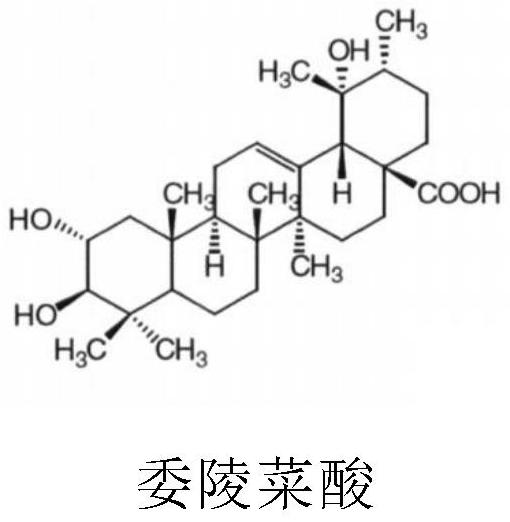
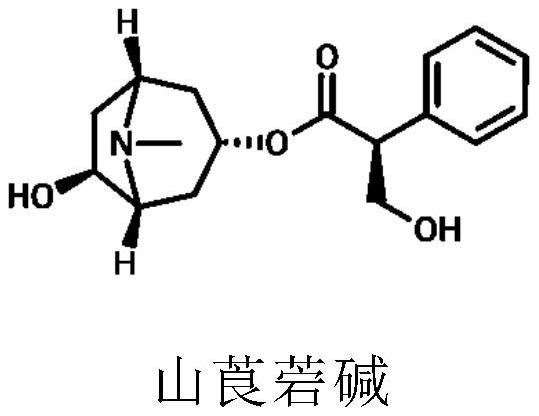
Application of tormentic acid and anisodamine capsule in preparation of medicine for treating diabetes
A technology of potentate acid and anisodamine, which is applied in capsule delivery, drug combination, metabolic diseases, etc., can solve the problems of slow drug dissolution rate and reduced drug efficacy, and achieve good dissolution, improved adaptability, and high stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
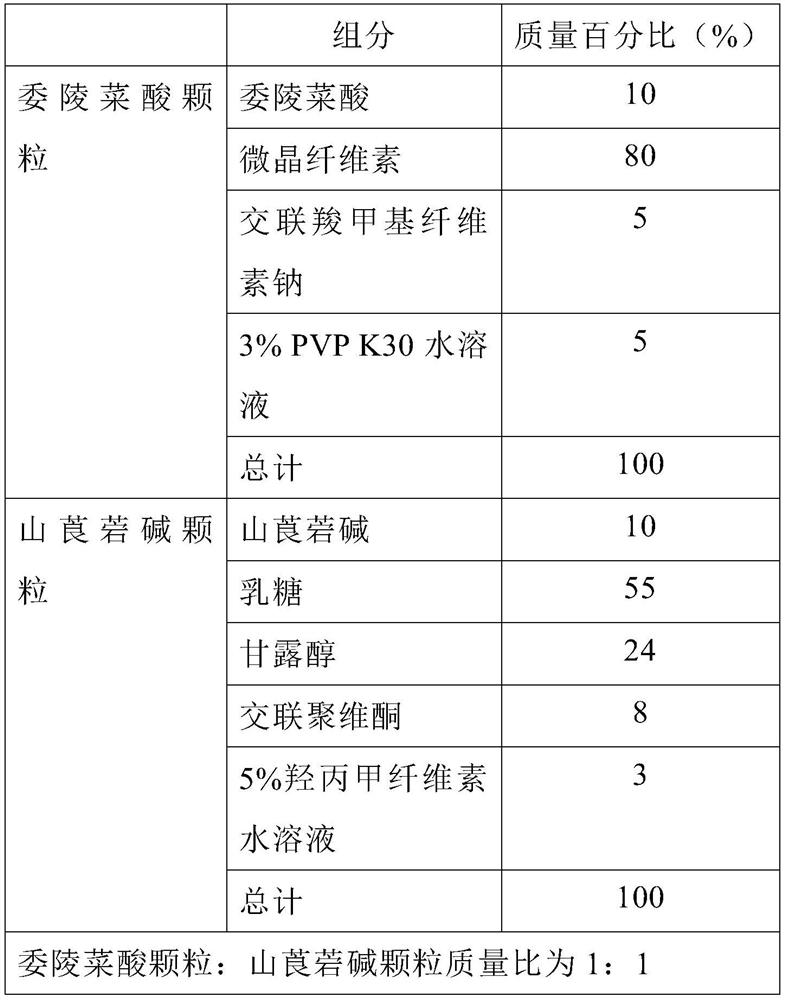
[0029] A kind of potentate and anisodamine capsule preparation, its prescription is composed as follows:
[0030]
[0031] The preparation method is as follows:
[0032] (1) Preparation of Pollentinic Acid Granules: Take Potentateic Acid, Microcrystalline Cellulose, and Croscarmellose Sodium by weight, each pass through a 100-mesh sieve, add a certain amount of 3% PVP K30 aqueous solution and mix Post-wet granulation.
[0033] (2) Preparation of anisodamine granules: take anisodamine, lactose, mannitol, and crospovidone by weight, each pass through a 100-mesh sieve, add a certain amount of 5% hypromellose aqueous solution, mix and wet French granulation.
[0034] (3) Pollentinic acid granules and anisodamine granules are mixed according to a mass ratio of 1:1, and then filled into capsules.
Embodiment 2
[0036] A kind of potentate and anisodamine capsule preparation, its prescription is composed as follows:
[0037]
[0038] The preparation method is as follows:
[0039] (1) Preparation of Pollentinic Acid Granules: Take Potentateic Acid, Microcrystalline Cellulose, and Croscarmellose Sodium by weight, each pass through a 100-mesh sieve, add a certain amount of 3% PVP K30 aqueous solution and mix Post-wet granulation.
[0040] (2) Preparation of anisodamine granules: take anisodamine, lactose, mannitol, and crospovidone by weight, each pass through a 100-mesh sieve, add a certain amount of 5% hypromellose aqueous solution, mix and wet French granulation.
[0041] (3) Pollentinic acid granules and anisodamine granules are mixed according to a mass ratio of 1:0.5, and then filled into capsules.
Embodiment 3
[0043] A kind of potentate and anisodamine capsule preparation, its prescription is composed as follows:
[0044]
[0045] The preparation method is as follows:
[0046] (1) Preparation of Pollentinic Acid Granules: Take Potentateic Acid, Microcrystalline Cellulose, Lactose, and Croscarmellose Sodium by weight, each pass through a 100-mesh sieve, and add a certain amount of 3% PVP K30 The aqueous solution is mixed and then wet granulated.
[0047] (2) Preparation of anisodamine granules: take anisodamine, lactose, and crospovidone by weight, each pass through a 100-mesh sieve, add a certain amount of 3% hypromellose aqueous solution, and wet granulate after mixing .
[0048] (3) Pollentin granules and anisodamine granules are mixed according to a mass ratio of 1:0.8, and then filled into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com