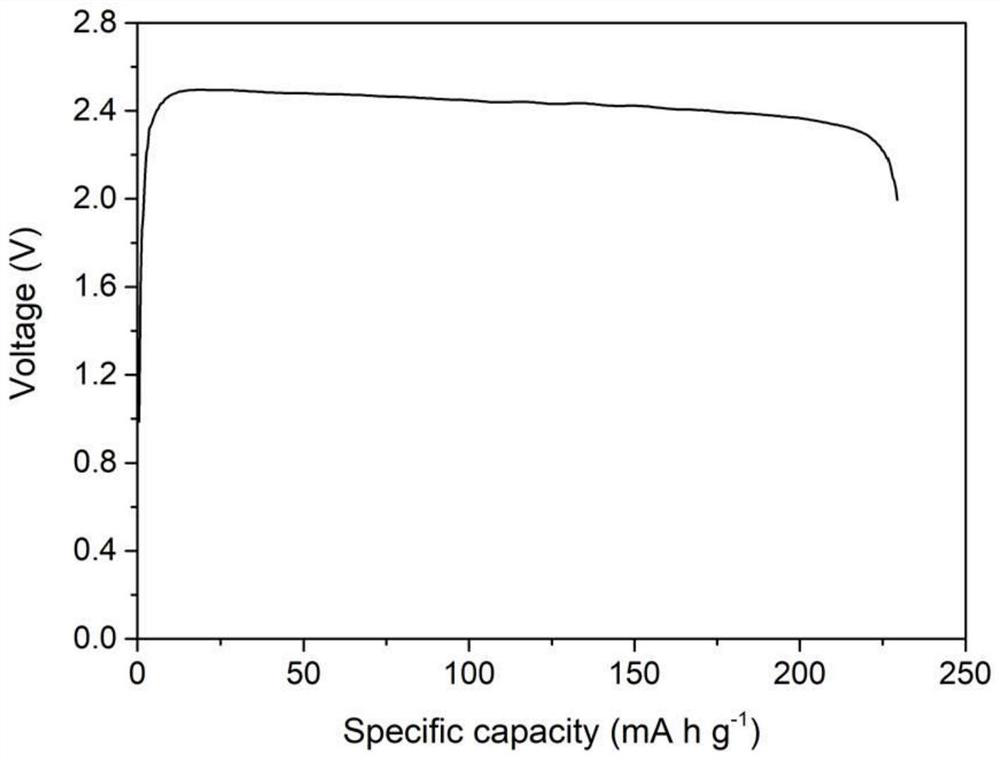
NiCl2 powder synthesized at low temperature and application
A powder and low-temperature technology, which is applied to low-temperature synthesized NiCl2 powder and its application field, which can solve the problems of low yield of chemical synthesis, limitation of practical application of NiCl2, long preparation period, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Weigh a portion of raw material NiCl 2 ·6H 2 O and one part extraction aid CuCl 2 2H 2 O, wherein the extraction aid is 5 at.% of the raw material, the two are dissolved in dehydrated alcohol, stirred evenly, and the quality of dehydrated alcohol is 4 times that of the raw material.
[0039] (2) The above solution was placed in a drying oven, the drying temperature was 100° C., the drying time was 8 hours, and the drying environment was atmospheric environment.
[0040] (3) Put the product obtained in step (2) into a muffle furnace for calcination, wherein the calcination temperature is 300°C, and the heating rate is 10°C·min -1 , Calcination time 3h. After the muffle furnace was cooled to room temperature, the sample was taken out, the surface product was scraped off, and the remaining product was sealed and stored.
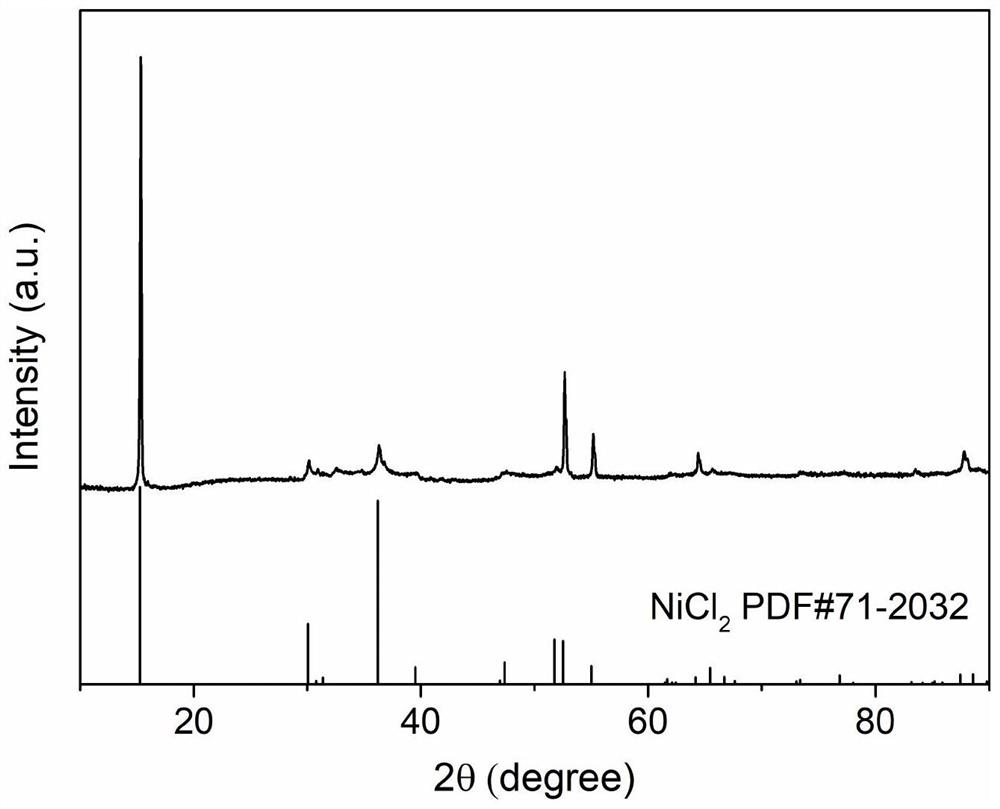
[0041] Show through XRD analysis, the NiCl prepared by above-mentioned steps 2 Corresponds well to anhydrous NiCl 2 The standard PDF card for ...
Embodiment 2
[0045] (1) Weigh a portion of raw material NiCl 2 ·6H 2 O and one part extraction aid CuCl 2 2H 2 O, wherein the extraction aid is 15 at.% of the raw material, the two are dissolved in dehydrated alcohol, stirred evenly, and the quality of dehydrated alcohol is 4 times that of the raw material.
[0046] (2) Put the above solution in a drying oven, the drying temperature is 80° C., the drying time is 16 hours, and the drying environment is atmospheric environment.
[0047] (3) Put the product obtained in step (2) into a muffle furnace for calcination, wherein the calcination temperature is 350°C, and the heating rate is 5°C·min -1 , Calcination time 6h. After the muffle furnace was cooled to room temperature, the sample was taken out, the surface product was scraped off, and the remaining product was sealed and stored.
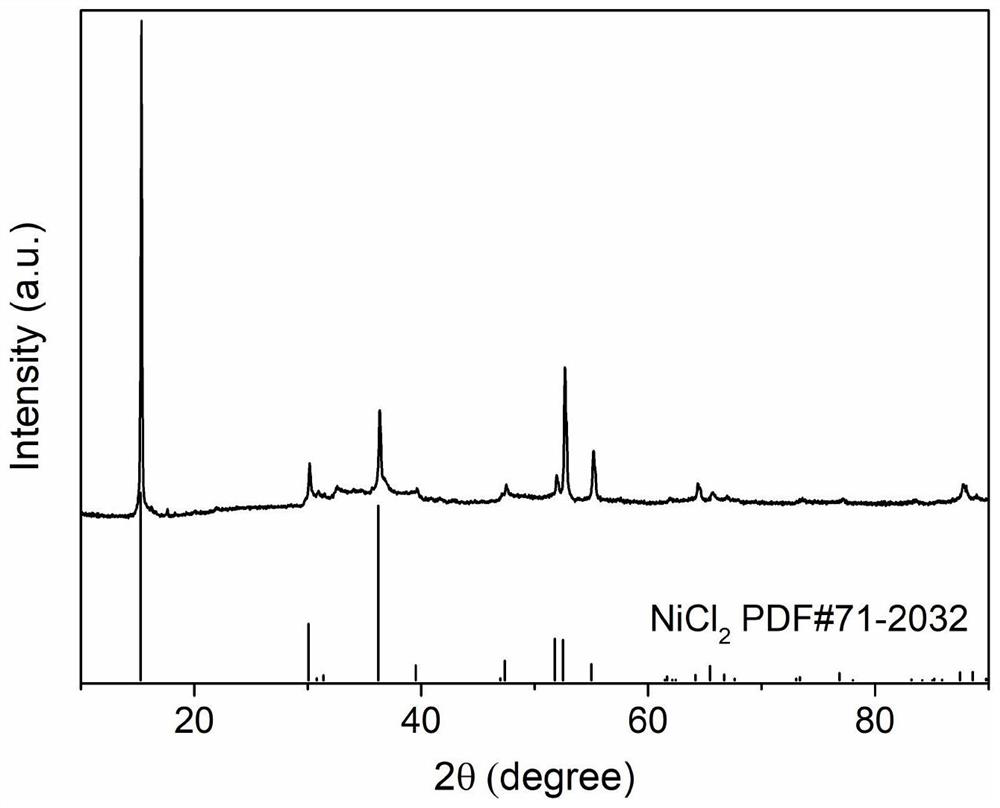
[0048] Show through XRD analysis, the NiCl prepared by above-mentioned steps 2 Corresponds well to anhydrous NiCl 2 The standard PDF card for the single...
Embodiment 3
[0052] (1) Weigh a portion of raw material NiCl 2 ·6H 2 O and a part of extraction aid CuCl, wherein the extraction aid is 3at.% of the raw material, the two are dissolved in absolute ethanol, stirred evenly, and the quality of absolute ethanol is 4.5 times that of the raw material.
[0053] (2) The above solution was placed in a drying oven, the drying temperature was 100°C, the drying time was 12 hours, and the drying environment was atmospheric environment.
[0054] (3) The product obtained in step (2) is placed in a muffle furnace for calcination, wherein the calcination temperature is 400°C, and the heating rate is 10°C·min -1 , Calcination time 3h. After the muffle furnace was cooled to room temperature, the sample was taken out, the surface product was scraped off, and the remaining product was sealed and stored.
[0055] Show through XRD analysis, the NiCl prepared by above-mentioned steps 2 Corresponds well to anhydrous NiCl 2 The standard PDF card for the single...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacity | aaaaa | aaaaa |
| Specific energy | aaaaa | aaaaa |
| Specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com