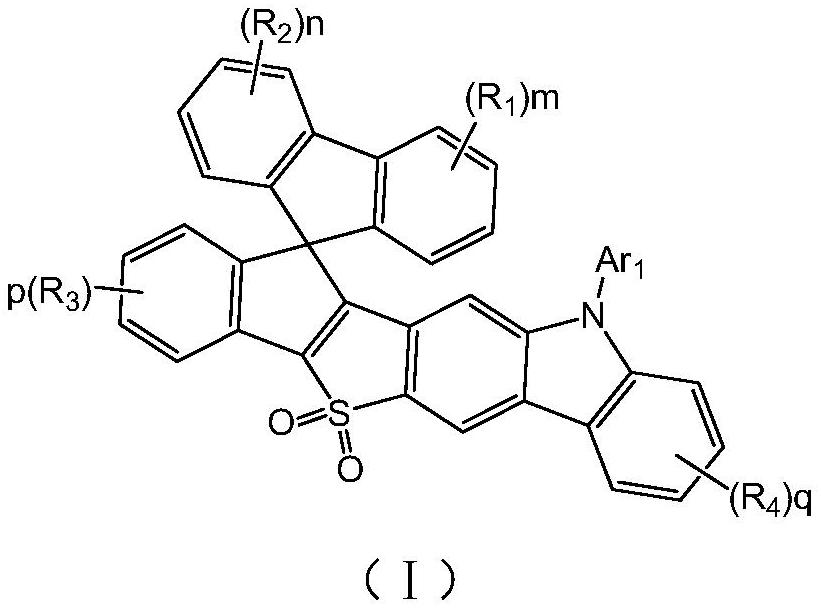
SO2-containing spiro pyrrolocarbazole compound and application thereof
A technology of rolocarbazoles and compounds, which is applied in the field of organic electroluminescence display, can solve problems such as triplet annihilation, triplet-polaron annihilation, and efficiency decline, so as to reduce driving voltage, improve photoelectric performance, Effect of High Film Stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044]According to the preparation method provided by the present invention, those skilled in the art can use known common means to realize, such as further selecting a suitable catalyst and solvent, determining a suitable reaction temperature, time, etc., which are not particularly limited in the present invention. The solvents, catalysts, bases and other raw materials used in the preparation process can be synthesized through open commercial channels or methods known in the art.
[0045] Using the preparation method provided by the invention, a series of compounds represented by general formula (I) were prepared.
[0046] Synthesis of Intermediates M1~M8
[0047] Synthesis of intermediate M1
[0048]
[0049] The synthetic route is as follows:
[0050]
[0051] The synthesis process comprises the following specific steps:
[0052] (1) Add 2-bromo-6-iodobiphenyl (35.8g, 0.10mol) and anhydrous tetrahydrofuran into a dry 1L three-necked flask under nitrogen protection....
Embodiment 1
[0085] The synthesis of embodiment 1 compound I-1
[0086]
[0087] The synthetic route is as follows:
[0088]
[0089] The preparation process includes: taking a 1L three-neck flask, equipped with magnetic stirring, adding potassium tert-butoxide (11.2g, 0.1mol), carbazole (16.7g, 0.1mol) and 400ml of toluene in sequence after nitrogen replacement; adding ( 0.4 g, 2 mmol) tri-tert-butylphosphine and (0.2 g, 1 mmol) palladium acetate. After the addition, heat up to 85°C; start to add dropwise a solution consisting of (64.7g, 0.1mol) M1 and 100ml of toluene, control the temperature at 80-120°C for 4 hours, and the reaction ends. Adjust to neutrality, separate the organic phase, extract, dry, perform column chromatography, and spin dry the solvent to obtain 61.52 g of light yellow solid with a yield of about 75%.
[0090] Product MS (m / e): 734.2; Elemental analysis (C 51 h 30 N 2 o 2S): theoretical value C: 83.36%, H: 4.11%, N: 3.81%; measured value C: 83.30%, H: 4....
Embodiment 2
[0091] Synthesis of Example 2 Compound I-14
[0092]
[0093] The synthetic route is as follows:
[0094]
[0095] The preparation process includes: adding M1 (64.7g, 0.1mol), 9-(4-(4,4,5,5-tetramethyl-1, 3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole (36.9g, 0.1mol), sodium carbonate (15.9g, 0.15mol), toluene 250mL, ethanol 150mL, water 100mL , the reaction system was protected by nitrogen replacement and added Pd(PPh 3 ) 4 (11.5g, 10mmol), magnetically stirred and heated to reflux reaction (system temperature 70-80°C) for 3 hours, and the reaction was stopped. The solvent was evaporated by subtraction, extracted with dichloromethane, dried over anhydrous magnesium sulfate, filtered, petroleum ether / ethyl acetate (2:1) column chromatography, spin-dried solvent, ethyl acetate beating, filtered to obtain 69.85g product I-14 , yield 86.2%.
[0096] Product MS (m / e): 810.23; Elemental analysis (C 57 h 34 N 2 o 2 S): theoretical value C: 84.42%, H: 4.23%, N: 3.45%; meas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com