A kind of synthesis method of dominant malachite green hapten and its application of hapten
A malachite green, synthetic method technology, applied in peptide preparation methods, chemical instruments and methods, cyanide reaction preparation, etc., can solve the problems of weak antibody specificity and sensitivity, and achieve high specificity and sensitivity, operation method Simple and fast, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
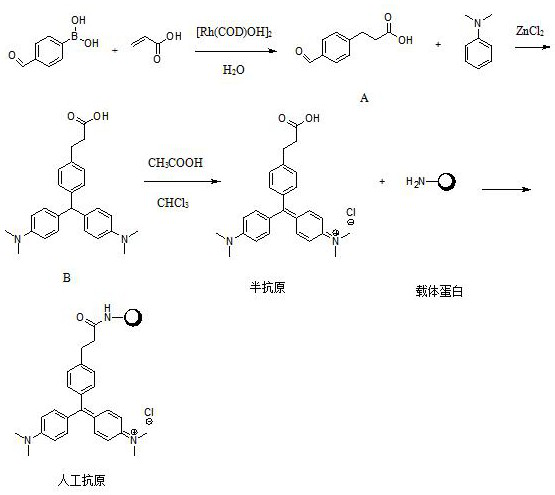
[0044] Dissolve 4-formylphenylboronic acid, acrylic acid and dimerized hydroxyl (1,5-cyclooctadiene) rhodium (I) at a molar ratio of 1:0.4:0.01 in 50ml degassed pure water, and replace the system with Ar protection , raised the temperature to 75°C and stirred for 16 hours, then lowered to room temperature, extracted the reaction solution 3 times with 60ml ethyl acetate, combined the organic phases, dried over anhydrous sodium sulfate, concentrated in vacuo, and applied the residue to silica gel column chromatography (CH 2 Cl 2 / EtOAc=9 / 1) to obtain compound A, namely 3-(4-formyl-phenyl)-propionic acid.
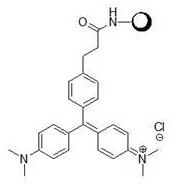
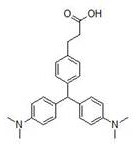
[0045] Put compound A, zinc chloride, and dimethylaniline with a molar ratio of 1:3:3 in 50ml of absolute ethanol, replace the system with Ar protection, heat to 90°C and stir for 24 hours. After the reaction is complete, concentrate the reaction solution , the residue was added with 25ml of methanol, acidified with 1M hydrochloric acid to pH = 3-4, and filtered to obtain lig...
Embodiment 2
[0048] The difference from Example 1 is that the molar ratio of 4-formylphenylboronic acid, acrylic acid and dimerized hydroxyl (1,5-cyclooctadiene) rhodium (I) in this example is 1:1:0.01.
[0049] Different from Example 1, the molar ratio of compound A, zinc chloride and dimethylaniline in this example is 1:3.5:3.
Embodiment 3
[0051] The difference from Example 1 is that the molar ratio of 4-formylphenylboronic acid, acrylic acid and dimerized hydroxyl (1,5-cyclooctadiene) rhodium (I) in this example is 1:0.6:0.01.
[0052] Different from Example 1, the molar ratio of compound A, zinc chloride and dimethylaniline in this example is 1:3.5:3.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com