Lactose powder bed three dimensional printing
A powder bed, lactose technology, used in organic active ingredients, pill delivery, additive processing, etc., can solve problems such as limiting the applicability of controlled release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0055] Lactose Characterization
[0056] All ingredients were commercially available from DFE Pharma GmbH, Germany, except povidone K30, which was obtained from Duchefa Farma.
[0057] All lactose grades for 3D printing are measured based on particle size as well as bulk and tap density.
[0058] Method used to determine particle size:
[0059] Particle sizing experiments were performed using a Helos laser diffraction unit, both from Synpatec GmbH (Clausthal-Zellerfeld, Germany), and Windox 5 software (5.10.0.04). The optical bench was equipped with an R5 Fourier lens for focusing the diffraction patterns of particles of the same size to the same location on the detector ring array. The lens covers a particle size range between 4.5 μm and 850 μm. Windox soft is performing the analysis according to the free mode in order to calculate the particle size distribution from the raw scattering data. Lactose powder was dispersed using a Rodos dry powder disperser and a Vibri d...
example 2
[0068] print square
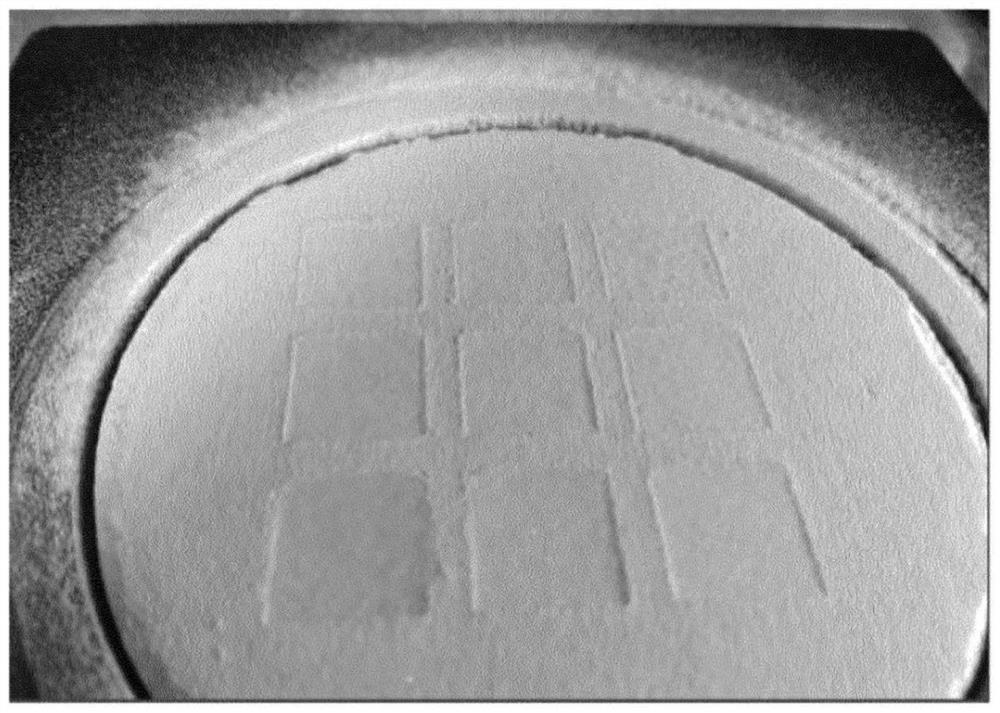
[0069] To assess whether a formulation is suitable for tablet printing, a square (one powder layer) can be printed and evaluated for wetting, consolidation and exudation. Square printing is performed as follows.
[0070] The formulations as given in the table were mixed on a roller set until homogeneous. Formulations were printed with a Sideswipe printer using Pronterface software. The powder blend was spread manually on the printing table with the help of a hand screen (700 microns) and rolled out automatically with rollers on the printer. A water / ethanol (95 / 5 vol. / vol.) mixture was sprayed over the powder bed in nine 1.4 cm 2 square. For each square, a different line spacing (as indicated in Table 3) was used, resulting in 9 different wetting squares.
[0071] Table 3: Print settings for printing squares in a single powder layer
[0072] sample Line distance(mm) Droplet / mm 1 0.2 5 2 0.25 4 3 0.3 3.3 4 0.35...
example 3
[0089] print tablet
[0090] The formulations as given in Table 8 were mixed on a roller set until a homogeneous mixture was obtained. Formulations were printed with a Sideswipe printer using Pronterface software.
[0091] The powder blend was spread manually on the printing table with the help of a hand screen (700 microns) and rolled out automatically with rollers on the printer. A water / ethanol (95 / 5 vol. / vol.) mixture was sprayed onto the powder using a 0.165 mm print head (Lee's) at 0.175 bar with a line spacing of 0.45 mm and at 2.22 drops / mm in a 9 mm circular shape. bed. Powder deposition and solution spraying were repeated 7 times to form flat tablets with a diameter of 9 mm and a height of 3 mm. The tablets were removed from the powder bed and dried in an oven at 50°C overnight. Suitable tablets of dimensions and masses as indicated in Table 9 were obtained.
[0092] Table 8: Tablet formulation (wt%)
[0093] 1 2 3 4 Lactohale 206 80 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com