Modified strain, application of modified strain in preparation of intestinal motility promoting preparation and product
An intestinal motility and strain technology, applied in the biological field, can solve the problems of obvious side effects, unstable effect, unclear mechanism, etc., and achieve the effects of reducing side effects, controllable expression, and precise control of drug delivery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
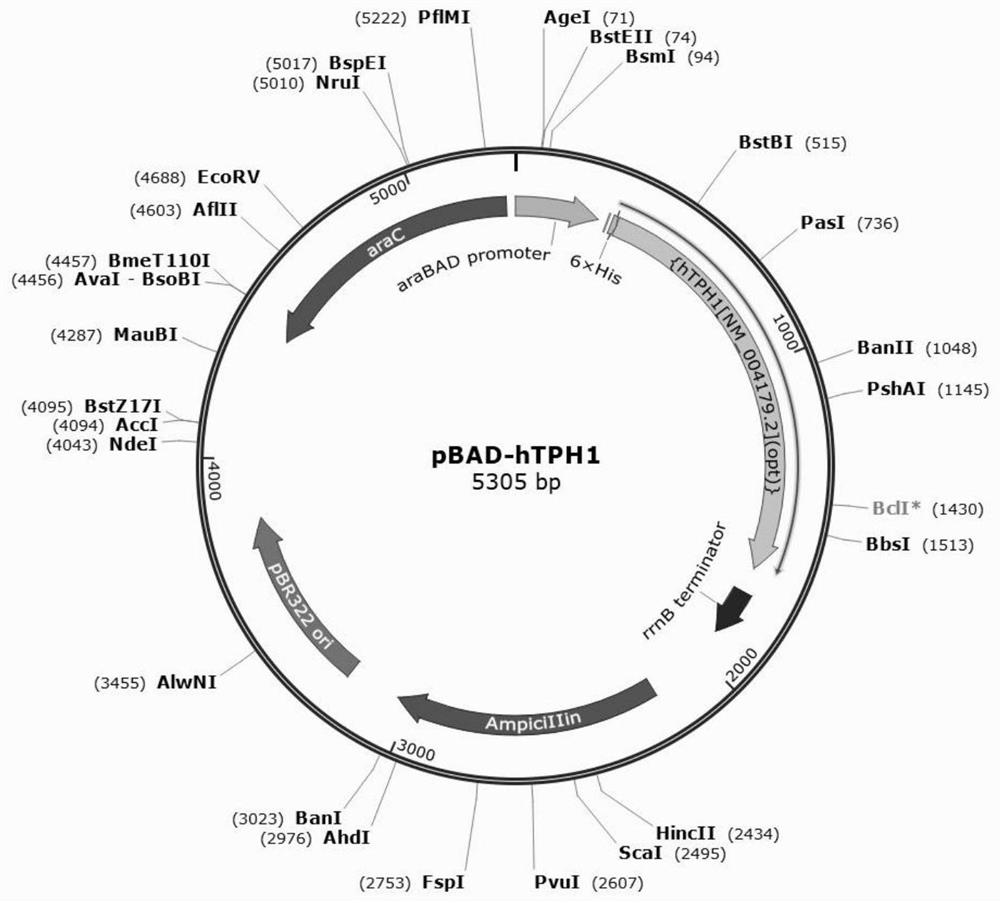
[0036] Embodiment 1 expression vector construction
[0037] The pBAD-hTPH1 expression vector is constructed according to the following method:
[0038] S1 Obtain the target gene fragment: obtain the target gene fragment by artificial synthesis according to the preferred expression sequence of tryptophan hydroxylase TPH1.
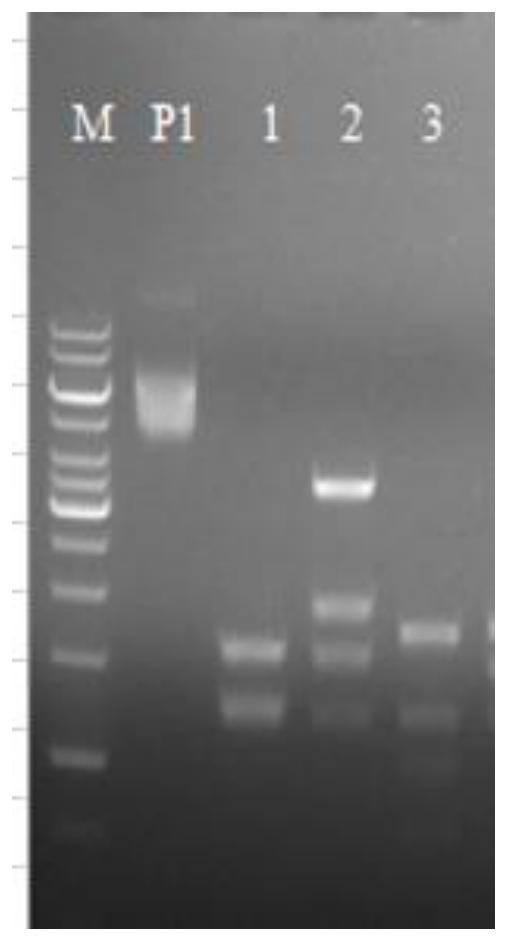
[0039] S2 Obtain the target vector fragment: Use restriction enzymes ApaLI and EcoRI to double digest the plasmid pBAD24. The enzyme digestion reaction system is: 10μLCutsmart, 2μLApaLI, 2μLECoRI, 2μg plasmamid, ddH 2 O less than 50 μL; 1% agarose gel electrophoresis detection after the reaction, such as figure 2 As shown, use the AxyprepDNA Gel Extraction kit 50-prep gel extraction kit to recover large fragments of the target vector fragment.
[0040] S3 to obtain the recombinant vector: using the GoldenGate cloning method, the reaction system: ApaLI-HF0.75ul, EcoRI-HF 0.75ul, 10×CutSmart TMBuffer 1ul, ATP (10mM) 1ul, T7 DNA ligase0.25ul, target gene fr...
Embodiment 2
[0042] Embodiment 2 obtains the transformation bacterial strain EcN pTPH bacterial strain
[0043] S1 recombinant vector construction: same as Example 1.
[0044] S2 Extract the recombinant vector: inoculate the successfully verified VB UltraStable strain containing the recombinant plasmid in 4 mL of LB culture medium containing ampicillin antibiotic (100 ng / mL), and culture at 37° C. and 180 rpm for 24 hours. Plasmids were extracted using the Novizan plasmid mini-prep kit.
[0045] Obtain the modified strain in S3: Electrotransform the recombinant plasmid obtained in S2 into EcN competent cells. The transformation method is carried out according to electrotransformation. After 1 h, the bacterial cells were obtained by centrifuging and concentrating. Spread the bacteria on the ampicillin antibiotic (100ng / mL) plate, culture for 24h, and obtain a positive strain, which is the modified strain.
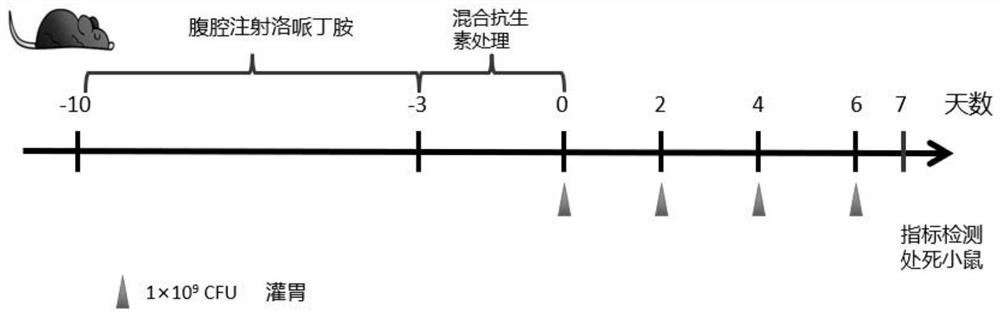
[0046] Following embodiment 3-5, schematic flow sheet is as figure 2 As shown, ...
Embodiment 3
[0049] Embodiment 3 Feces water content test
[0050] After the end of gavage, put the mice individually into a cage lined with absorbent paper, collect the feces of 3 mice, weigh them as the wet weight, and after freeze-drying, it is the dry weight, according to the following formula:
[0051] Water content of feces = water content of feces (%) = (wet weight of feces - dry weight of feces) / wet weight of feces × 100, calculate the water content of feces, and take the average value.
[0052] The result is as image 3 As shown in Table 1:
[0053] Table 1 Fecal water content test results
[0054] normal group(%) Model group (%) EcN wild type (%) EcNpTPH (%) 66.86909 50.26911 57.54977 59.33918 68.03653 53.16117 55.73154 61.59484 66.5937 54.73606 54 61.87923
[0055] Shows: normal group, model group, EcN wild-type bacteria group, EcN pTPH bacteria group feces moisture content are: 67.15%, 52.72%, 55.76%, 60.94%
[0056] It can be seen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com