Small molecules binding cyclin-dependent kinase inhibitor 1b(p27kip1)
A compound, the technology of R31, is applied in the field of compounds targeting inherently disordered proteins, which can solve problems such as difficulties in design methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0189] For the preparation of solutions or suspensions, for example water, especially sterile water, or physiologically acceptable organic solvents such as alcohols (ethanol, propanol, isopropanol, 1,2-propylene glycol, polyethylene glycol, Glycols and their derivatives, fatty alcohols, partial esters of glycerol), oils (e.g., peanut oil, olive oil, sesame oil, almond oil, sunflower oil, soybean oil, castor oil, cowshoe oil), paraffin, dimethylmethylene Sulfone, triglyceride, etc.
[0190] In the case of liquid dosage forms such as potable solutions, the following substances can be used as stabilizers or solubilizers: lower aliphatic monovalent and polyvalent alcohols with 2 to 4 carbon atoms, such as ethanol, n-propanol, glycerol, Polyethylene glycols with a molecular weight between 200-600 (for example, 1%-40% in water), diethylene glycol monoethyl ether, 1,2-propanediol, organic amides, such as aliphatic C1-C6-carboxylic acids and ammonia or amides of primary, secondary or...
Embodiment
[0346] Having now generally described the embodiments of the disclosure, the following examples describe some other embodiments of the disclosure. While embodiments of the disclosure are described in conjunction with the following examples and corresponding text and figures, it is not intended to limit the aspects of the disclosure to this description. On the contrary, the intention is to cover all alternatives, modifications, and equivalents as included within the spirit and scope of embodiments of the present disclosure.
[0347] method
[0348] protein preparation
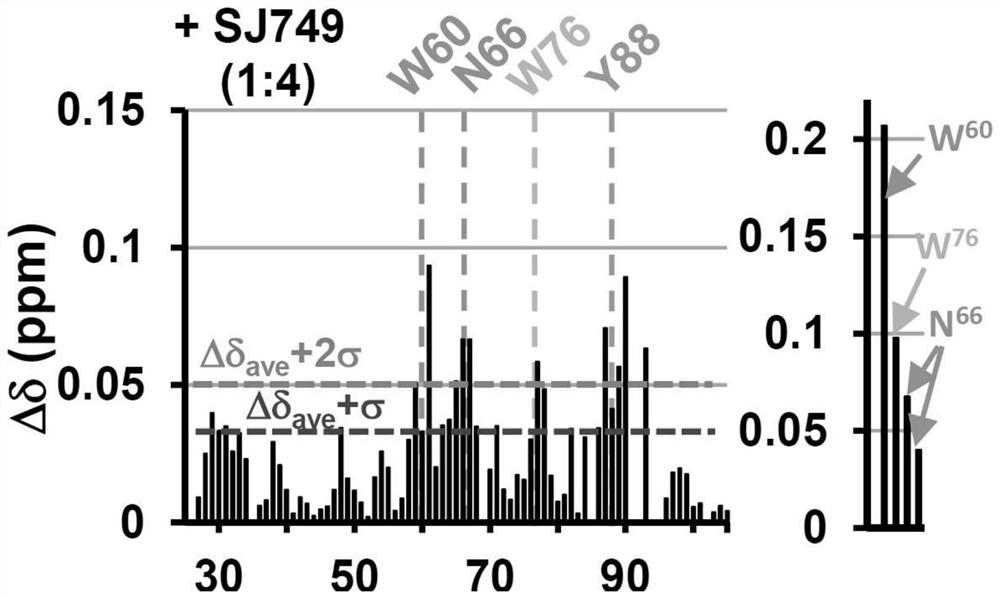
[0349] After subcloning into pET28a (Novagen) using established procedures, the p27 construct containing an N-terminal 6xHis affinity tag was expressed in E. coli (BL21 / DE3) (Lacy, E.R. et al., Nat Struct Mol Biol, 2004, Volume 11, Chapter 4: pp. 358-364). This includes p27-KID (residues 22-105 of human p27) and p27-D2 (residues 58-105 of human p27) and the following mutants: W60A, W76A, W60A-W76A. Isotopi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com