A co-production process of desalted intestinal mucosal protein powder and heparin
A technology for intestinal mucosa and protein powder is applied in the field of co-production of desalted intestinal mucosa protein powder and heparinoid, which can solve problems such as environmental protection and production and operation problems, and achieve the effect of reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A desalted intestinal mucosal protein powder co-production process with heparin, including the following characteristic steps:
[0019] The process steps for desalting intestinal mucosal protein powder are:
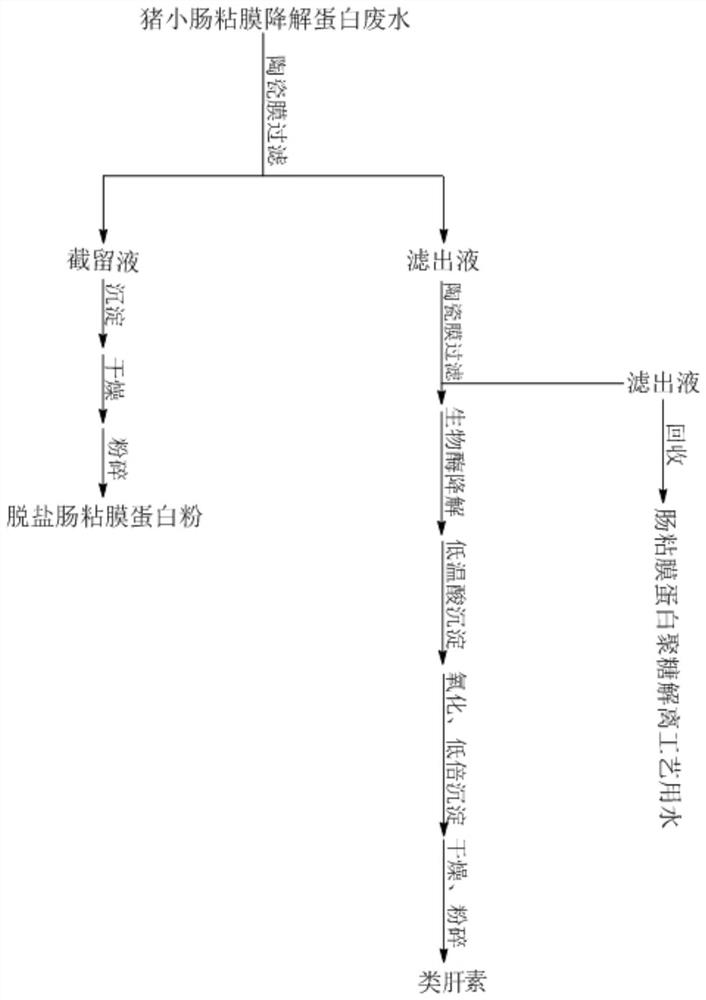
[0020] S1, collect 500L of salt-containing protein wastewater in the production process of crude heparin sodium as raw material liquid, transfer the raw material liquid to the turnover box, circulate and filter through the ceramic membrane with a pore size of 10KD, and add purified water to the turnover tank during the filtration process to keep the raw material liquid volume unchanged, and detect the change in salinity of the raw material liquid. When the salinity of the detected raw material liquid drops below 0.1%, no more purified water is added to the turnover tank, and the volume of the feedstock liquid is 100L, that is, the interception solution, which is the concentrated desalted intestinal mucosal protein solution;
[0021] S2, to step S1 obtained in the conce...
Embodiment 2
[0039] A desalted intestinal mucosal protein powder co-production process with heparin, including the following characteristic steps:
[0040] The process steps for desalting intestinal mucosal protein powder are:
[0041] S1, collect 500L of salt-containing protein wastewater in the production process of crude heparin sodium as raw material liquid, transfer the raw material liquid to the turnover box, circulate and filter through the ceramic membrane with a pore diameter of 30KD, add purified water to the turnover tank during the filtration process to keep the raw material liquid volume unchanged, and detect the change in the salinity of the raw material liquid. When the salinity of the detected raw material liquid drops below 0.1%, no more purified water is added to the turnover tank, and the volume of the feedstock liquid is 100L, that is, the interception solution, which is the concentrated desalted intestinal mucosal protein solution;
[0042] S2, to step S1 obtained in the c...
Embodiment 3
[0057] A desalted intestinal mucosal protein powder co-production process with heparin, including the following characteristic steps:
[0058] The process steps for desalting intestinal mucosal protein powder are:
[0059] S1, collect 500L of salt-containing protein wastewater in the production process of crude heparin sodium as raw material liquid, transfer the raw material liquid to the turnover box, circulate and filter through the ceramic membrane with a pore diameter of 30KD, add purified water to the turnover tank during the filtration process to keep the raw material liquid volume unchanged, and detect the change in the salinity of the raw material liquid. When the salinity of the detected raw material liquid drops below 0.1%, no more purified water is added to the turnover tank, and the volume of the feedstock liquid is 100L, that is, the interception solution, which is the concentrated desalted intestinal mucosal protein solution;
[0060] S2, to step S1 obtained in the c...
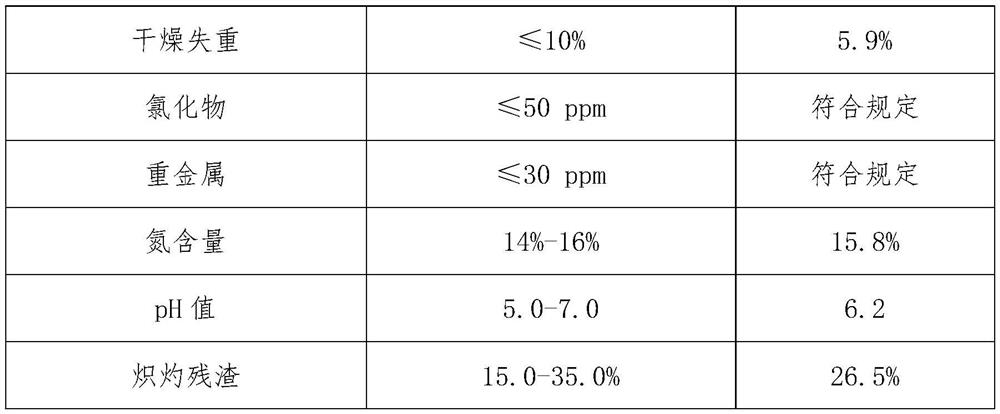
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com