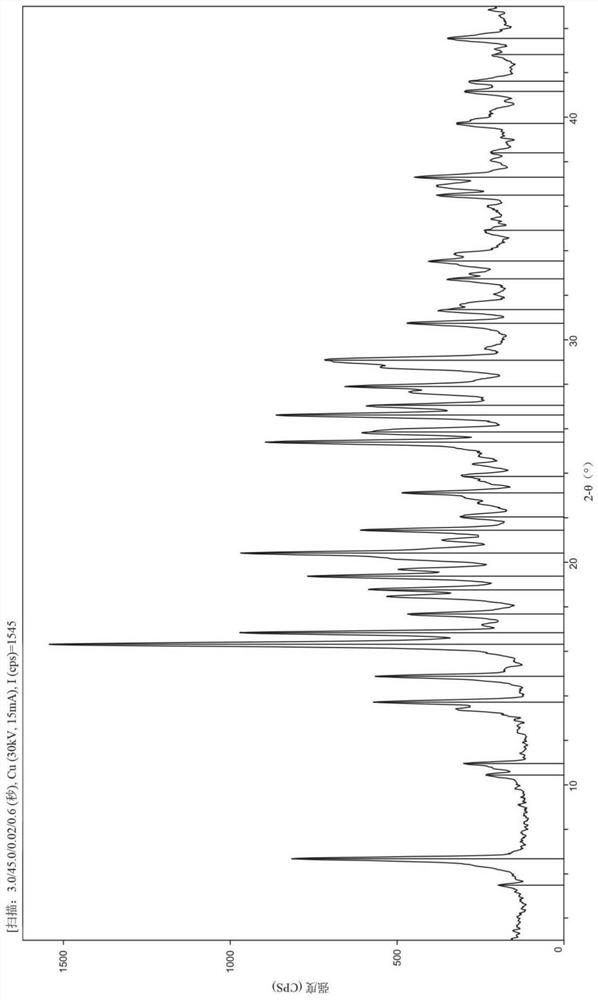
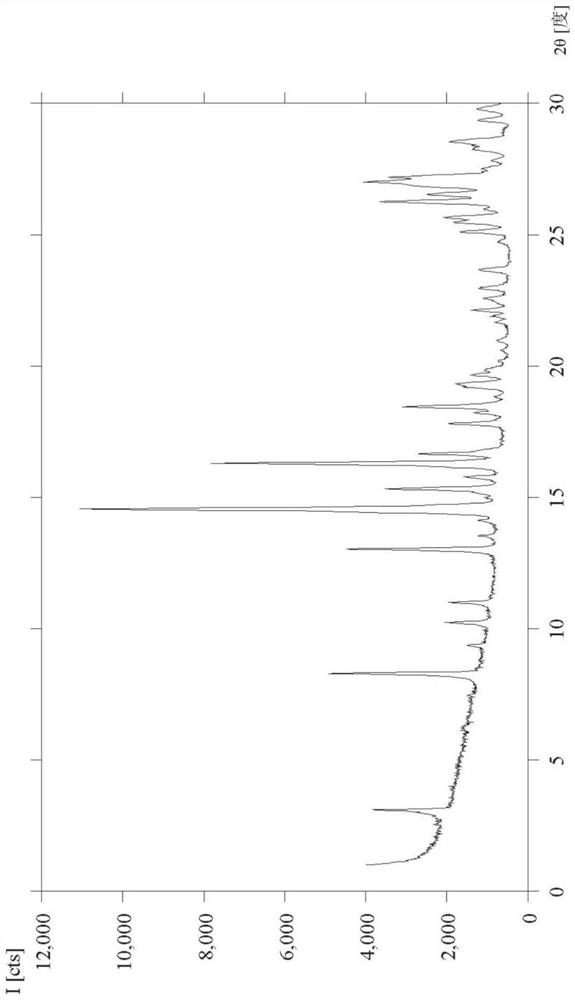
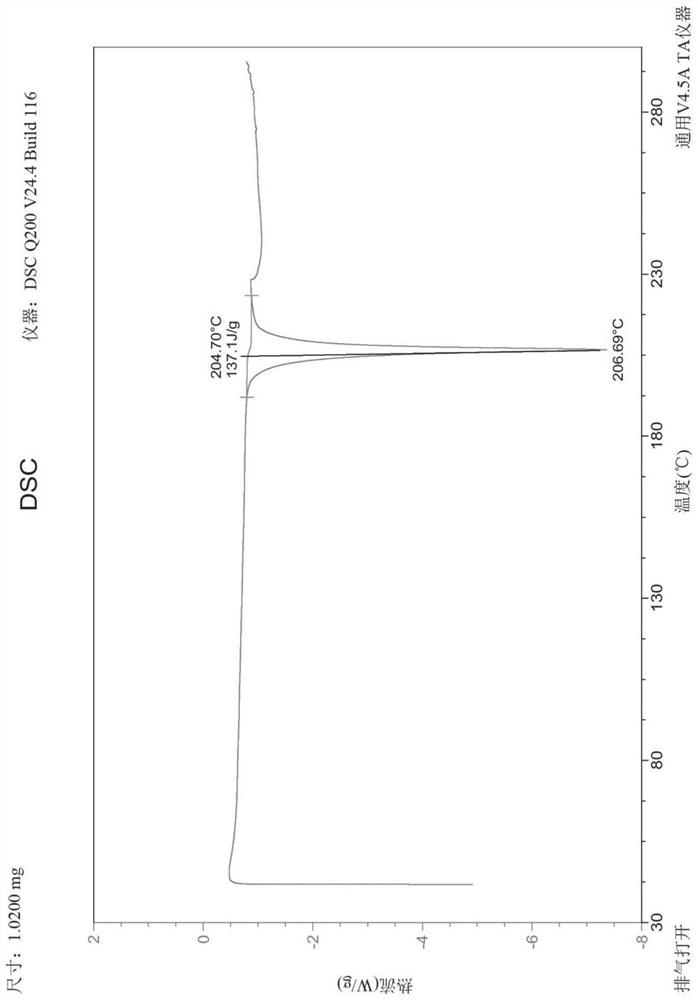
Selective inhibitor of protein arginine methyltransferase 5 (PRMT5)
A technology of hydrochloride and maleate, which can be used in medical preparations containing active ingredients, organic active ingredients, drug combinations, etc., and can solve problems such as gene expression repression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0081] A more complete understanding of the present disclosure can be obtained by reference to the following description including the following definitions and examples. Certain features of the disclosed compositions and methods that are described herein in the context of separate aspects may also be provided in combination in a single aspect. Alternatively, various features of the disclosed compositions and methods which are, for brevity, described in the context of a single aspect, may also be provided separately or in any subcombination.
[0082] "Pharmaceutically acceptable" means that which is or can be approved by a regulatory agency of the Federal or a state government, or its counterpart in a country other than the United States, or listed for use in the United States Pharmacopoeia or other generally recognized pharmacopoeia. For use by animals (eg, humans).
[0083] "Pharmaceutically acceptable salt" refers to a salt of a compound of the present disclosure that is p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com