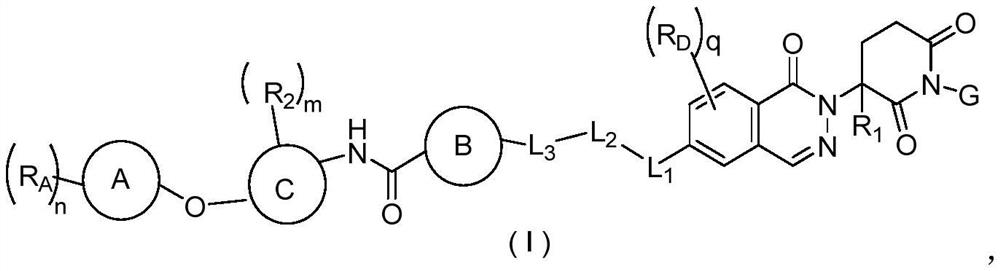
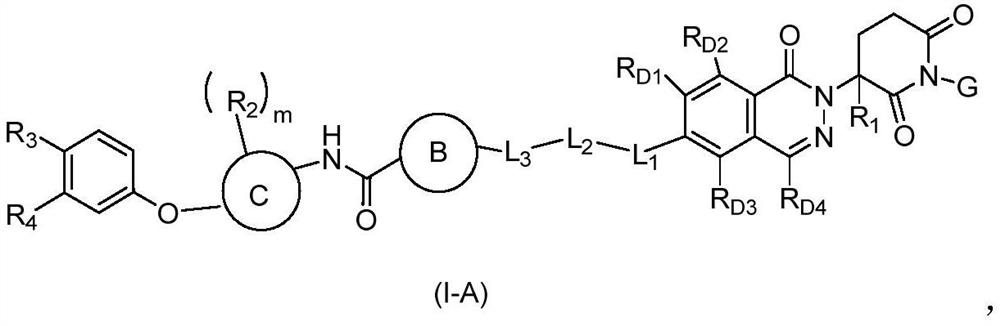
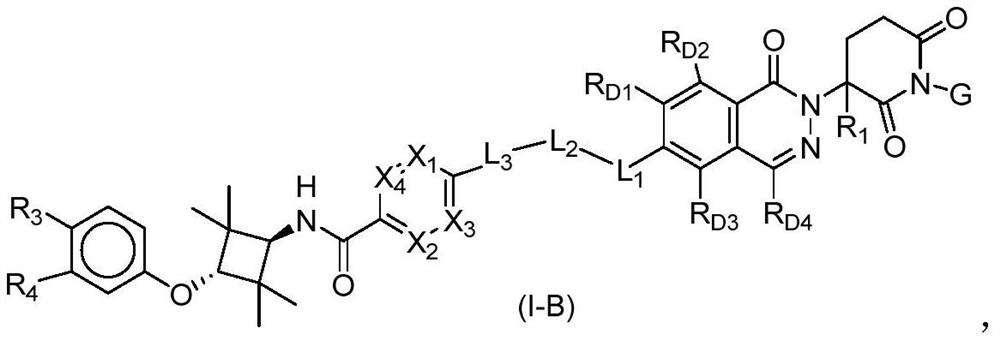
Phthalazinone compound as well as preparation method and medical application thereof
A technology of compounds and medicinal effects, which is applied in the field of phthalazinone compounds and their preparation and medical application, and can solve problems such as not necessarily applicable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0165] Reference Example 1: Preparation of Intermediate I-1
[0166]
[0167] 2-Bromo-4-fluorobenzoic acid (7.00 g, 31.9 mmol) was dissolved in thionyl chloride (30.0 mL), and N,N-dimethylformamide (0.25 mL) was added. The reaction solution was stirred at 80° C. for 2 hours under the protection of nitrogen. The reaction solution was cooled to room temperature, and after removing thionyl chloride under reduced pressure, the residue was dissolved in dichloromethane (100 mL). Diethylamine (11.7 g, 159.8 mmol) was added, and the reaction solution was stirred at room temperature overnight. The reaction solution was washed with saturated aqueous sodium bicarbonate (30.0 mL), water (30.0 mL), saturated brine, dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure to obtain the crude product of intermediate I-1, which was directly used in the next reaction without purification.
[0168] LC-MS(ESI)[M+H] + 274.0.
[0169] 1 H NMR...
reference example 2
[0170] Reference Example 2: Preparation of Intermediate I-2
[0171]
[0172] Intermediate I-1 (8.50g), potassium vinylfluoroborate (4.98g, 37.2mmol) and potassium carbonate (10.7g, 77.5mmol) were dissolved in dioxane (80.0mL) / water (20.0mL) To the mixed solution, bistriphenylphosphinepalladium dichloride (1.09g, 1.55mmol) was added. The reaction solution was stirred overnight at 90° C. under nitrogen protection. The reaction solution was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to obtain a residue. The residue was dissolved in ethyl acetate (100.0 mL), washed successively with water (30.0 mL), washed with saturated brine, dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure to obtain the crude product of intermediate I-2, which was directly used in the next reaction without purification.
[0173] LC-MS(ESI)[M+H] + 222.2.
[0174] 1H NMR (400MHz, Chloroform-d) δ7.2...
reference example 3
[0175] Reference Example 3: Preparation of Intermediate I-3
[0176]
[0177] Intermediate I-2 (7.00g) was dissolved in a mixed solvent of dioxane (70.0mL) / water (30.0mL), and potassium osmate dihydrate (466.0mg, 1.27mmol) and sodium periodate ( 13.5 g, 63.2 mmol). The reaction was stirred at room temperature for 2 hours. The reaction solution was filtered, and the filtrate was concentrated to obtain a residue. The residue was dissolved in ethyl acetate (100.0 mL), washed successively with water (30.0 mL), washed with saturated brine, dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated to obtain a residue, and the residue was separated and purified by silica gel chromatography to obtain intermediate I-3.
[0178] 1 H NMR (400MHz, Chloroform-d) δ9.99 (d, J = 2.3Hz, 1H), 7.63–7.57 (m, 1H), 7.38–7.28 (m, 2H), 3.59 (q, J = 7.1Hz, 2H), 3.11(q, J=7.1Hz, 2H), 1.27(t, J=7.1Hz, 3H), 1.02(t, J=7.1Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com