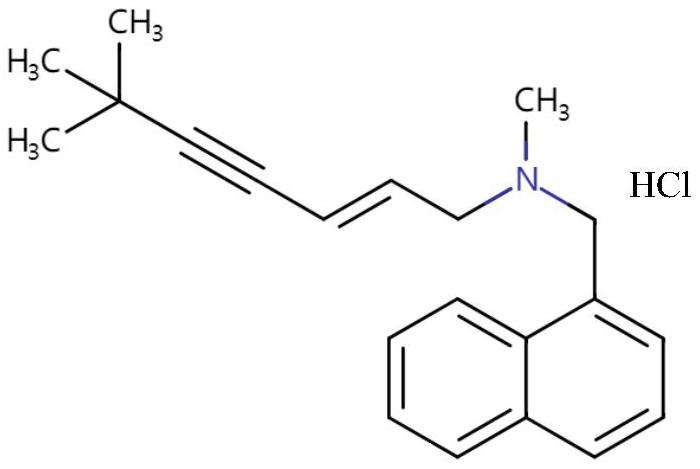
Terbinaphthol hydrochloride gel and preparation method thereof
A technology for terbinafine hydrochloride and gel, which is applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of reduced gel adhesion, error-prone mixing sequence, Complex preparation process and other problems, to achieve the effect of increasing concentration gradient, promoting transdermal absorption, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1) Prescription
[0045]
[0046] 2) Preparation of
[0047] (A) the prescribed amount of hydrochloric acid than naphthol Laid uniformly dispersed in 20, a mixture of sunflower oil and Tween 1.2 propylene glycol;
[0048] (B) the mixture in (a) was added with stirring in purified water containing a disodium hydrogen phosphate, mixed, allowed to stand for 1h to achieve phase equilibrium, resulting microemulsion drug;
[0049] (C) the amount of gellan gum formulation is uniformly dispersed in hot water (purified water, b) 80 ℃ and stirred to dissolve, cooled down to below 50 ℃;
[0050] And (d) all of the drug was transferred to a microemulsion gellan gum aqueous solution, stir, cooled to room temperature to obtain terbinafine hydrochloride phenol gel.
Embodiment 2
[0052] 1) Prescription
[0053]
[0054] 2) Preparation of
[0055] (A) the prescribed amount of hydrochloric acid than naphthol Laid uniformly dispersed in 20, a mixture of sunflower oil and Tween 1.2 propylene glycol;
[0056] (B) the mixture in (a) was added with stirring in purified water containing a disodium hydrogen phosphate, mixed, allowed to stand for 1h to achieve phase equilibrium, resulting microemulsion drug;
[0057] (C) the amount of gellan gum formulation is uniformly dispersed in hot water (purified water, b) 80 ℃ and stirred to dissolve, cooled down to below 50 ℃;
[0058] And (d) all of the drug was transferred to a microemulsion gellan gum aqueous solution, stir, cooled to room temperature to obtain terbinafine hydrochloride phenol gel.
Embodiment 3
[0060] 1) Prescription
[0061]
[0062]
[0063] 2) Preparation of
[0064] (A) the prescribed amount of hydrochloric acid than naphthol Laid uniformly dispersed in 20, a mixture of sunflower oil and Tween 1.2 propylene glycol;
[0065] (B) the mixture in (a) was added with stirring in purified water containing a disodium hydrogen phosphate, mixed, allowed to stand for 1h to achieve phase equilibrium, resulting microemulsion drug;
[0066] (C) the amount of gellan gum formulation is uniformly dispersed in hot water (purified water, b) 80 ℃ and stirred to dissolve, cooled down to below 50 ℃;
[0067] And (d) all of the drug was transferred to a microemulsion gellan gum aqueous solution, stir, cooled to room temperature to obtain terbinafine hydrochloride phenol gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com