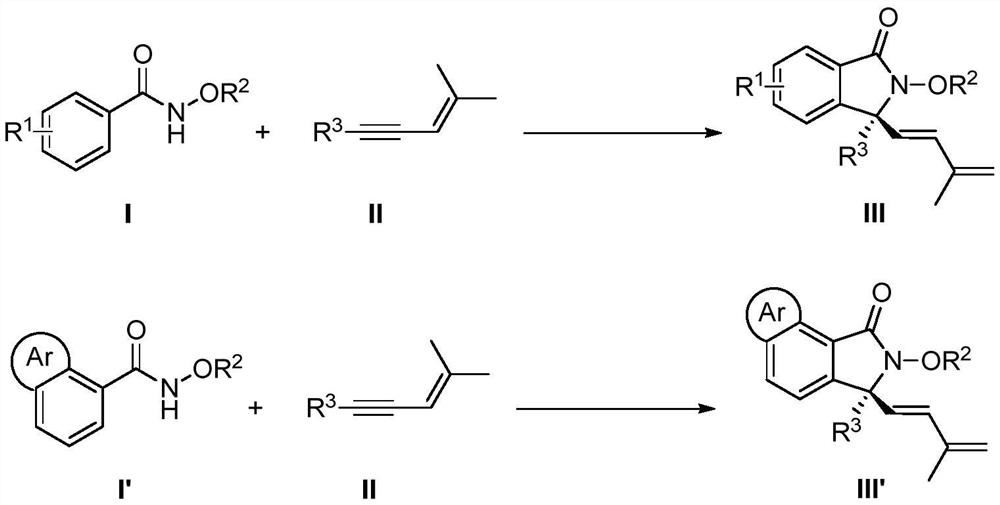
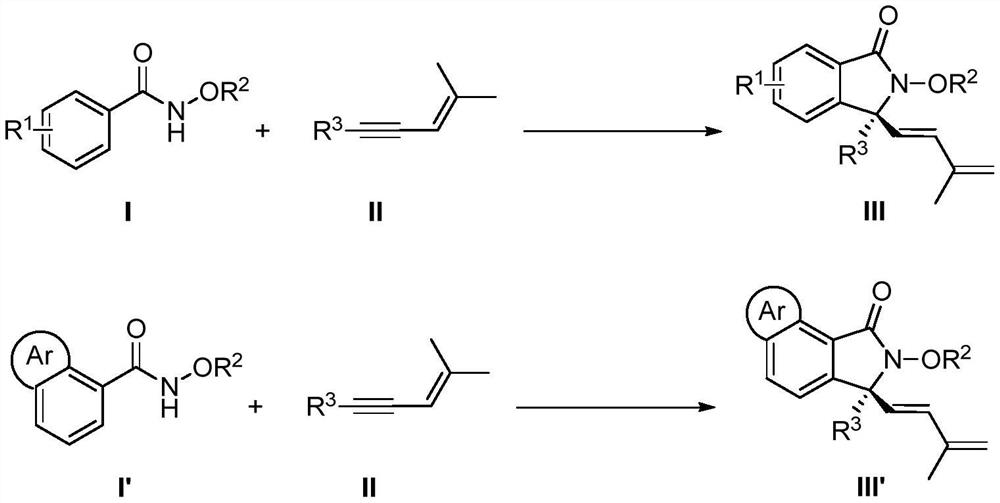
Synthesis method of chiral 3, 3-disubstituted isoindolinone compound through rhodium catalysis
A technology for isoindolinone and compounds, which is applied in the field of synthesis of chiral 3,3-disubstituted isoindolinone compounds, can solve the problems that the cyclization reaction has not yet been realized, and achieve a wide range of substrates, The effect of mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
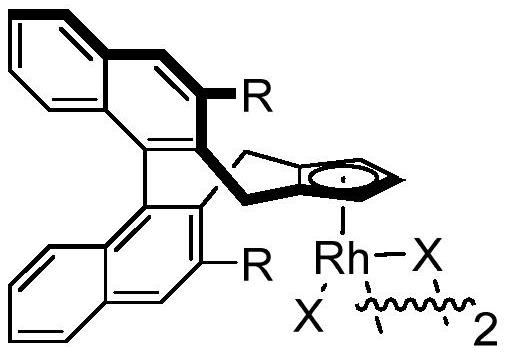
[0021] Add 15.1mg (0.1mmol) N-methoxybenzamide, 5.0mg (0.004mmol) chiral rhodium catalyst A, 35.0mg (0.24mmol) silver difluoride to the test tube, cool to -15°C, add 2 mL of 3-pentanol, 18.0 mg (0.15 mmol) (4-methylpent-3-en-1-ynyl) cyclopropane, and 200 μL of 1.0 mol / L acetic acid in 3-pentanol solution, stirred at 10 ° C React for 72 hours. After the reaction is over, add 1.0 mL of ethylenediamine to quench, and spin dry to obtain the crude product. The crude product is quickly passed through the column with silica gel (petroleum ether:ethyl acetate=10:1~5:1) to obtain the following structural formula: Yellow oily liquid 18.8mg, its yield is 70%, its ee value is 90% according to HPLC analysis.
[0022]
[0023] The structural characterization data of the resulting product are: 1 H NMR (600MHz, CDCl 3 )δ7.84(d, J=7.8Hz,1H),7.53(td,J=7.8,1.2Hz,1H),7.46(td,J=7.2,0.6Hz,1H),7.25(d,J=7.2 Hz,1H),6.61(d,J=16.2Hz,1H),5.70(d,J=15.6Hz,1H),5.05(s,1H),5.04(s,1H),4.05(s,3H), 1.82(s...
Embodiment 2
[0025] In this example, the N-methoxybenzamide in Example 1 is replaced with equimolar N-methoxy-4-methylbenzamide, and the other steps are the same as in Example 1 to obtain a colorless The oily liquid was 24.5 mg, the yield was 86%, and the ee value was 95% according to HPLC analysis.
[0026]
[0027] The structural characterization data of the resulting product are: 1 H NMR (600MHz, CDCl 3 )δ7.72(d, J=7.8Hz, 1H), 7.26(d, J=8.4Hz, 1H), 7.02(s, 1H), 6.62(d, J=16.2Hz, 1H), 5.69(d, J=15.6Hz,1H),5.06(s,1H),5.05(s,1H),4.03(s,3H),2.44(s,3H),1.83(s,3H),1.57–1.53(m,1H ),0.73–0.68(m,1H),0.60–0.56(m,1H),0.49–0.45(m,1H),-0.02–-0.05(m,1H); 13 C NMR (150MHz, CDCl 3 )δ164.6, 144.0, 142.5, 141.1, 135.5, 129.6, 127.4, 127.0, 123.64, 123.59, 118.1; [α] D 20 =+52.8 (c=0.1, CHCl 3 ).
Embodiment 3
[0029] In this example, the N-methoxybenzamide in Example 1 is replaced with equimolar N-methoxy-4-tert-butylbenzamide, and the other steps are the same as in Example 1 to obtain the following structural formula: The color oily liquid was 28.4 mg, the yield was 87%, and the ee value was 95% according to HPLC analysis.
[0030]
[0031] The structural characterization data of the resulting product are: 1 H NMR (600MHz, CDCl 3)δ7.76(d, J=8.4Hz, 1H), 7.49(dd, J=7.8, 1.8Hz, 1H), 7.20(d, J=1.2Hz, 1H), 6.63(d, J=15.6Hz, 1H), 5.71(d, J=16.2Hz, 1H), 5.06(s, 1H), 5.05(s, 1H), 4.03(s, 3H), 1.84(s, 3H), 1.60–1.56(m, 1H ),1.34(s,9H),0.73–0.68(m,1H),0.57–0.53(m,1H),0.49–0.44(m,1H),-0.05–0.09(m,1H); 13 CNMR (150MHz, CDCl 3 )δ164.6, 155.8, 143.5, 141.1, 135.5, 127.4, 127.0, 125.9, 123.4, 119.9, 118.1, 70.2, 65.0, 35.3, 31.3, 18.5, 15.8, 2.4, 0.4; HRMS (ESI-TOF) (m / z) :C 21 h 27 NNaO 2 ,([M+Na] + ), theoretical value 348.1934, measured value 348.1935; [α] D 20 =-8.4 (c=0.1, CHCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com