Nucleoside compound and preparation method thereof
A technology of compounds and nucleosides, which is applied in the field of nucleoside compounds and their preparation, and can solve problems such as the inactivation of xanthine nucleosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
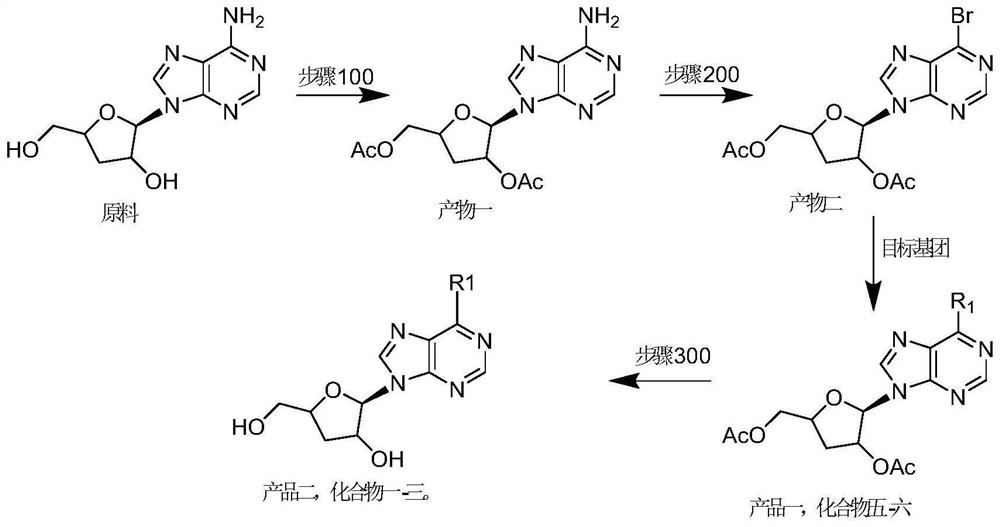
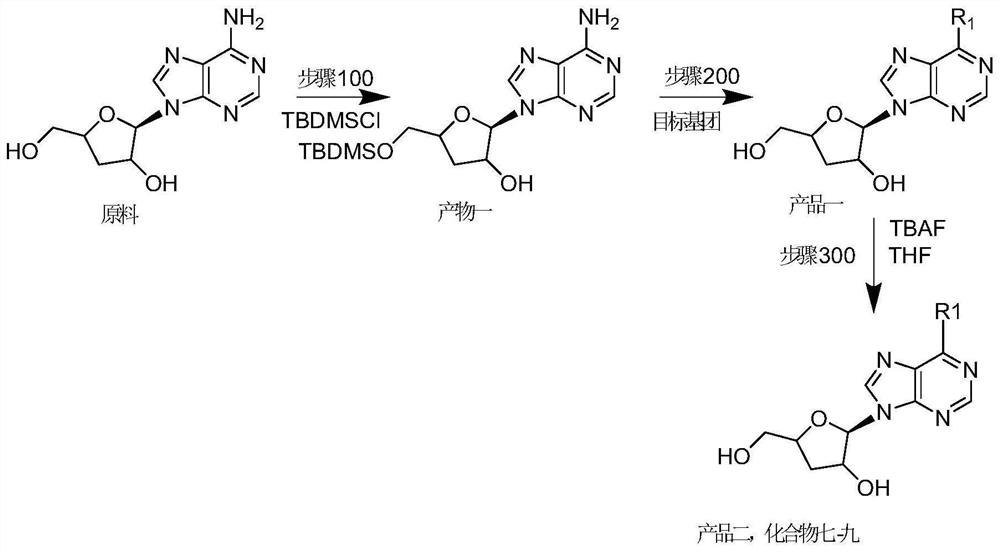
[0042] In addition, this embodiment also provides a method for preparing nucleoside compounds, including: step 100, using 3'-deoxyadenosine nucleoside as a raw material, performing substituent protection to obtain a product one; step 200, the Product 1, introducing the target group to obtain Product 1; or, after the step 200 "introducing the target group to the product 1 to obtain Product 1", it also includes: Step 300, removing the product 1 Protection reaction to obtain product two; wherein, said product one or said product two are nucleoside compounds as described above.
[0043] As mentioned above, 3'-deoxyadenosine is the raw material drug-cordycepin. As mentioned above, step 100 is for the protection of 2'-OH and / or 5'-OH in 3'-deoxyadenosine nucleoside, and step 200 introduces the target group as for 6-NH 3 Substitution reactions of substituents introduce corresponding groups. Step 300 correspondingly removes the protection of 2'-OH and / or 5'-OH in step 100 to obtain ...
specific Embodiment approach 1
[0082] Further, specific embodiment 1: the antibacterial plane 112 includes a detachable substrate 1122, a gel layer 1123 disposed on the substrate, and a gel layer 1123 located away from the detachable substrate 1122. The microarray assembly 1124 on one side; the microarray assembly 1124 includes a plurality of microneedle monomers 1124a arranged at equal intervals on its surface; The drug delivery pipeline 1124-2 connected to the drug delivery thorn 1124a-1, and the inclusion gel 1124-3 coated on the periphery of the drug delivery pipeline 1124-2; the drug delivery thorn 1124a-1 And antibacterial components are set in the drug delivery pipeline 1124-2;
[0083] As mentioned above, the drug delivery puncture head 1124a-1 can pierce the cuticle of the human facial skin, and administer the drug into the skin cuticle, and the drug enters the skin through the drug delivery pipeline 1124-2 and the drug delivery thorn head 1124a-1, In order to achieve the antibacterial effect agai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com