Olefin hydroformylation method
A technology for hydrogenation and formylation of olefins, applied in chemical instruments and methods, carbon monoxide reaction preparation, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of increasing catalyst stability, cumbersome synthesis steps, and reactivity low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
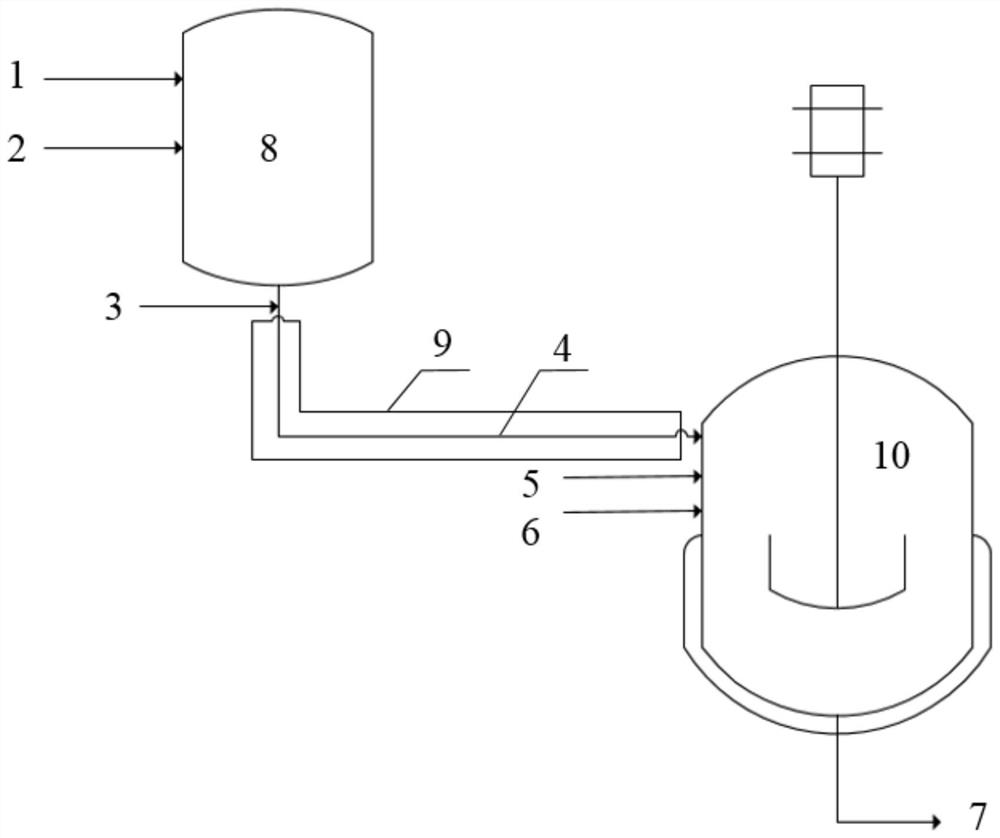
[0046] Such as figure 1 As shown, the 1-octene hydroformylation process for preparing nonanal is as follows: using rhodium acetylacetone dicarbonyl as the main catalyst, 2,2-bis[(diphenylphosphino)methyl]-1,1-bis[(diphenylphosphino)methyl]-1,1- Benzene (BISBI) is the ligand, the molar ratio of the main catalyst (calculated as rhodium) to the ligand is 1:5, and in terms of total moles, the molar ratio of 1-octene:Rh is 1000:1, and the concentration of Rh It is 1.6mmol / L. The closed reaction system was filled with N 2 After purging, use syngas (CO:H 2 =1:1) to replace several times and open the temperature control system of the system to maintain the temperature of the whole system at 80°C, the synthesis gas (CO:H 2 =1:1) into the gas-liquid mixer and the reactor respectively and keep the pressure in the gas-liquid mixer at 1.0MPa and the pressure in the reactor at 1.5MPa. Add toluene to the gas-liquid mixer to make toluene and synthesis gas (CO:H 2 =1:1) mixing, the reside...
Embodiment 2
[0048] The experimental method is the same as in Example 1, except that the pressure in the gas-liquid mixer is changed to 0.5 MPa, and the rest of the experimental conditions are unchanged. The test results are shown in Table 1.
Embodiment 3
[0050] The experimental method is the same as in Example 1, except that the pressure in the gas-liquid mixer is changed to 0.25 MPa, and the rest of the experimental conditions are unchanged. The test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com