Preparation method of 1, 4-dimethyl pentylamine hydrochloride
A technology of dimethylpentylamine hydrochloride and dimethylformylpentylamine, which is applied in 1 field, can solve the problems of complex preparation process, many raw material components, low yield and the like, and achieves simple preparation method and easy process control. , produce cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
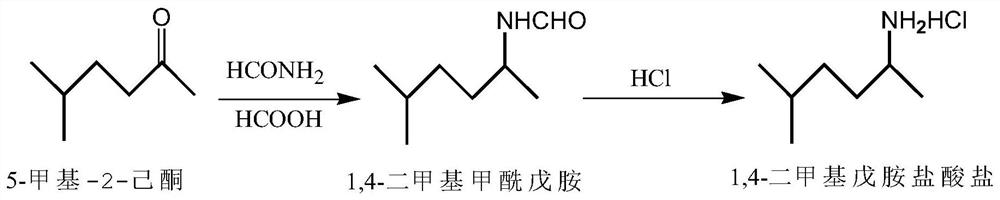
[0024] A kind of preparation method of 1,4-dimethylamylamine hydrochloride, the reaction process of its preparation method is as figure 1 As shown, the specific steps are:
[0025] (1) Amidation: Use 5-methyl-2-hexanone as raw material, add reducing agent formic acid and ammonium formate while stirring, heat up to 110-115°C for 3 hours, then heat up to 130-135°C for 3 hours hours, and finally raised the temperature to 150-160° C. for 3 hours, cooled, stood to separate layers, washed, dried, and suction filtered to obtain 1,4-dimethylformyl amylamide.
[0026] (2) Hydrolysis into salt: adding the above-mentioned 1,4-dimethylformyl amylamide into hydrochloric acid with a mass concentration of 36%, heating and reflux reaction under stirring, distillation under reduced pressure, standing for stratification, collecting the lower layer material for suction filtration, After crystallization and drying, 1,4-dimethylpentylamine hydrochloride was obtained.
[0027] A kind of preparati...
Embodiment 1
[0033] A kind of preparation method of 1,4-dimethylamylamine hydrochloride is:
[0034] (1) Amino acylation: Add 11.4 g (0.1 mol) of 5-methyl-2-hexanone into a 500 mL four-neck flask, add 4.6 g (0.1 mol) of formic acid and 7.6 g (0.12 mol) of ammonium formate while stirring. Raise the temperature to 110°C for 3 hours, then raise the temperature to 130°C for 3 hours, and finally keep it at 155°C for 3 hours. Cool to about 40°C and add 40g of water. Stir for 30 minutes, stand to separate the layers, wash the oil layer three times with saturated brine, add anhydrous sodium sulfate to dry overnight, and filter with suction to obtain 11.6g of 1,4-dimethylformyl amylamide oil with a yield of 89.9%, which was directly used in Next reaction.
[0035] (2) Hydrolysis into salt: Add 11.6 g (0.09 mol) of 1,4-dimethylformyl amylamide and 29 g of 36% hydrochloric acid into a 500 mL four-necked flask. The temperature was raised to reflux under stirring for 10 hours. Distill under reduced...
Embodiment 2
[0037] A kind of preparation method of 1,4-dimethylamylamine hydrochloride is:
[0038] (1) Amino acylation: Add 11.4 g (0.1 mol) of 5-methyl-2-hexanone into a 500 mL four-necked flask, and add 6.0 g (0.13 mol) of formic acid and 8.8 g (0.14 mol) of ammonium formate while stirring. Raise the temperature to 115°C for 3 hours, then raise the temperature to 135°C for 3 hours, and finally keep it at 160°C for 3 hours. Cool to about 40°C and add 40g of water. Stir for 30 minutes, stand to separate the layers, wash the oil layer three times with saturated brine, add anhydrous sodium sulfate to dry overnight, and filter with suction to obtain 11.3g of 1,4-dimethylformyl amylamide oil with a yield of 87.6%, which was directly used in Next reaction.
[0039] (2) Hydrolysis into salt: Add 11.3 g (0.088 mol) of 1,4-dimethylformyl amylamide and 33 g of 36% hydrochloric acid into a 500 mL four-necked flask. The temperature was raised to reflux under stirring for 10 hours. Distill under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com