Methods of producing polypeptides using a cell line resistant to apoptosis
A cell line and cell technology, applied in the field of cell line and cell culture, can solve the problem of few HEK293 cells found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0190] The present invention will be more fully understood by reference to the following examples. However, these examples should not be considered as limiting the scope of the invention. It should be understood that the examples and embodiments described herein are for illustrative purposes only, and that various modifications or changes therefrom are suggested to those skilled in the art and are to be included within the spirit and purview of this application and the accompanying within the scope of the patent application.
example 1
[0191] Example 1: Generation and testing of a more stable HEK293 cell line
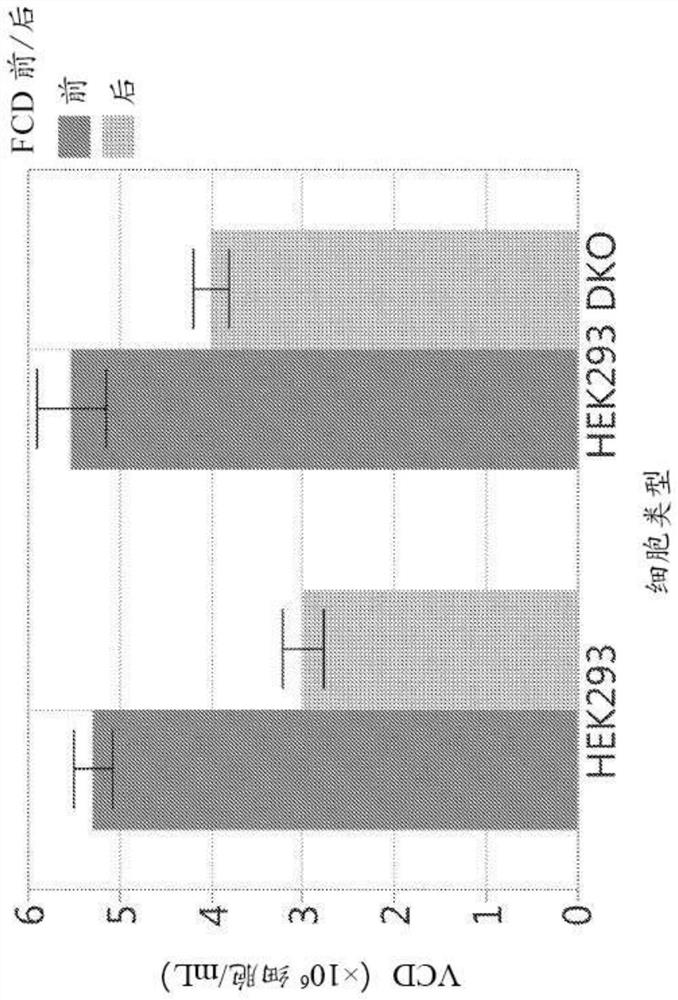
[0192]As described above, HEK293 cells are sensitive to shear stress and tend to develop low viability when cultured in bioreactors. It is assumed that cell lines resistant to apoptosis will exhibit higher productivity and more stable performance in bioreactors. An antiapoptotic HEK293 cell line (HEK293 DKO) was engineered to delete the pro-apoptotic genes Bax and Bak by using zinc finger nuclease technology (Cost et al., (2010) Biotechnol. Bioeng. 105:330-340). During apoptosis, Bax and Bak permeate the mitochondrial membrane, eventually causing activation of the caspase protein, which triggers programmed cell death (Taylor et al., (2008) Nat. Rev. Mol. Cell Biol. 9:231-241) . Deletion of Bax and Bak in CHO cell lines has previously been shown to correlate with higher culture survival and transfection titers (Macaraeg et al., (2013) Biotechnol. Prog. 29:1050-1058). Suppression or deletion of Bax a...
example 2
[0224] Example 2: Scale-up of HEK293 seed cultures
[0225] To efficiently generate cell populations to support large-scale (10L) transfection, HEK293 DKO seed cultures were grown in 35L controlled bioreactors rather than multiple shake flasks. A regularly subcultured (i.e., split every 3 to 4 days) seed culture bioreactor with a working volume of 20 to 35 L will provide sufficient 6 40 to 70 L of transfected cells were seeded per cell / mL. This enables high-throughput, large-scale transient production runs to be performed on a regular basis.
[0226] Different pH and dissolved oxygen (DO) controlled conditions were first evaluated in two 2L bioreactors before testing HEK293 DKO seed cultures in 35L bioreactors. Bioreactor #1 used a pH set point of 7.0 with an insensitivity zone ±0.03 and a DO set point of 30%; these are typical conditions for CHO stable cell line cultures (Li et al., (2010) mAbs.2:466- 477; Li et al., (2012) Biotechnol. Bioeng. 109:1173-1186; Yuk et al., (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com