Hydroxychloroquine sulfate hydrate as well as crystal form, preparation method and application thereof
A technology of hydroxychloroquine sulfate and hydrate, applied in the field of hydroxychloroquine sulfate hydrate, can solve problems such as poor hygroscopicity, and achieve the effects of stable and reliable quality, simple and feasible preparation method, and good prospect of finished medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1: the preparation of hydroxychloroquine sulfate amorphous
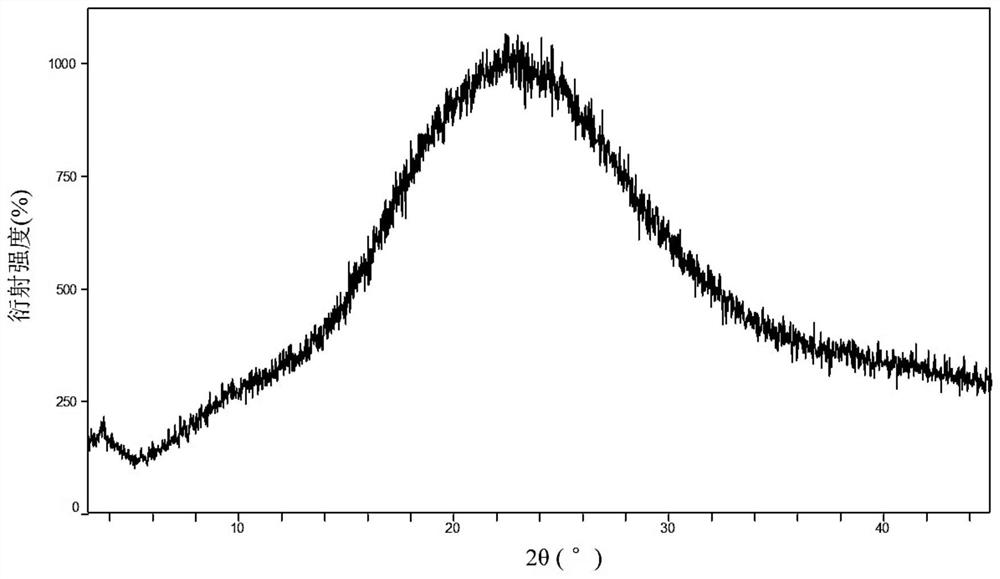
[0089] Take 3g of crystal form A of hydroxychloroquine sulfate (synthesized according to Example 2 of patent CN108727263A), add 9mL of 2,2,2-trifluoroethanol and stir to dissolve, place it on a rotary evaporator and steam it, and set the temperature to 60 °C, the rotational speed was set at 100 rpm, and the title product was obtained after spin-drying. The XRPD detection product is an amorphous state, and its XRPD spectrum is as follows figure 1 shown.
Embodiment 2
[0090] Embodiment 2: Preparation of the crystal form B of hydroxychloroquine sulfate monohydrate
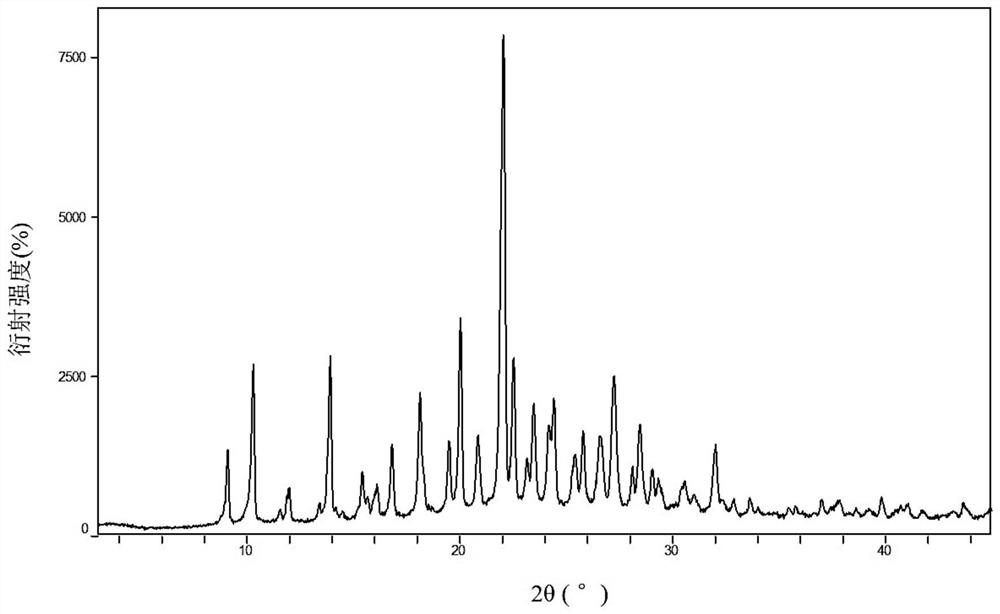
[0091] Take 300 mg of amorphous hydroxychloroquine sulfate prepared in Example 1, add m-xylylene dimethyl ether 8 mL (water containing impurities, water content is 0.1 wt%), place on a shaker, 25 ° C, 250 rpm, shake for 48 hours and then filter. The filtered sample was dried in a vacuum oven (vacuum degree -0.1 MPa) at 35 °C for 24 h to obtain the title product. The product detected by XRPD is crystal form B, and the peak width and peak height relative intensity in the X-ray powder diffraction pattern represented by 2θ angle are as follows:
[0092]
[0093]
[0094] XRPD spectrum as figure 2 shown.
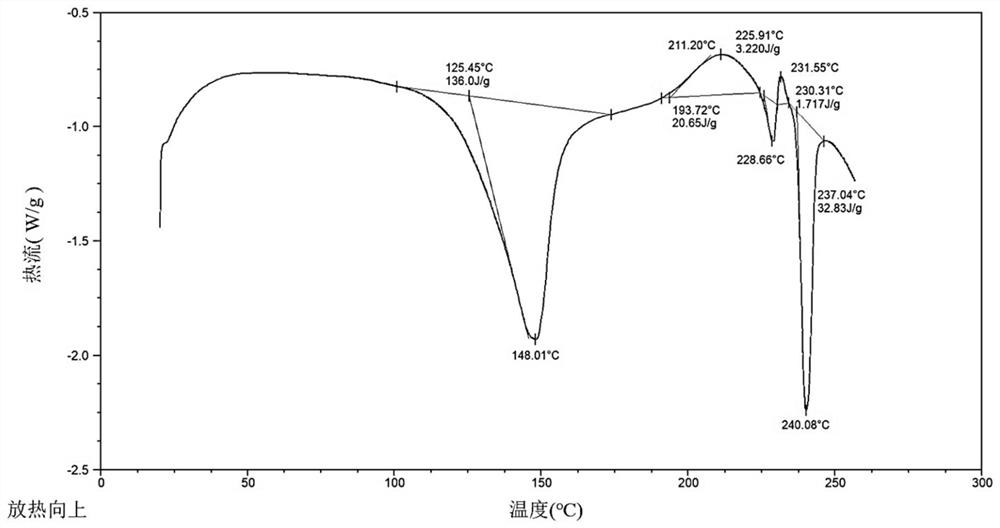
[0095] DSC spectrum such as image 3 shown.
[0096] TGA spectrum as Figure 4 As shown, it shows that the corresponding weight loss of the sample is 4.140% before 150°C. According to the molecular weight of hydroxychloroquine sulfate, the ratio of hydroxychloroquine sul...
Embodiment 3
[0099] Embodiment 3: the preparation of the crystal form B of hydroxychloroquine sulfate monohydrate
[0100] Take 300 mg of amorphous hydroxychloroquine sulfate prepared in Example 1, add 8 mL of 3-methyl-1-butanol (water containing impurities, water content is 0.1 wt%), place on a shaker, 25 ° C, 250 rpm, and shake for 24 h After filtering. The filtered sample was dried in a vacuum oven (vacuum degree -0.1 MPa) at 35 °C for 24 h to obtain the title product. The product detected by XRPD is the crystal form B of hydroxychloroquine sulfate monohydrate, and its identification data is basically the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com