Application of urine-derived stem cells in preparation of biological preparation and medicine for treating non-alcoholic fatty liver disease, losing weight and lowering lipid
A non-alcoholic, biological preparation technology, applied in the field of prevention and treatment of drugs, to achieve the effect of inhibiting lipid deposition and glucose tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: USCs reduce the increase in liver weight caused by high fat.
[0018] 1.1 Experimental method
Embodiment approach 1
[0019] Embodiment 1: USCs inhibit high-fat diet-induced hepatic lipid deposition
[0020] 1.1 Experimental method:
[0021] 1.1.1 Isolation and culture of USCs
[0022] In order to study the effects of USCs in various diseases, the present invention has successfully established a complete set of methods for USCs isolation, culture, subculture, and identification, and has isolated and cultured more than 60 cases of human-derived urinary stem cells.
[0023] (1) Urine collection: clean the urethral opening, and collect 200 mL of clean midstream urine (non-morning urine). (Definition of mid-segment urine: mid-segment urine refers to clinical urine culture. When urine is collected, the urethra is washed clean by the first urine, and the middle urine is taken as a sample for culture. The purpose is to prevent urine from being polluted. Here, The special medium for USCs was prepared as follows: serum-free keratinocyte medium (K-SFM) and precursor cell medium (EFM) were mixed accor...
Embodiment 2
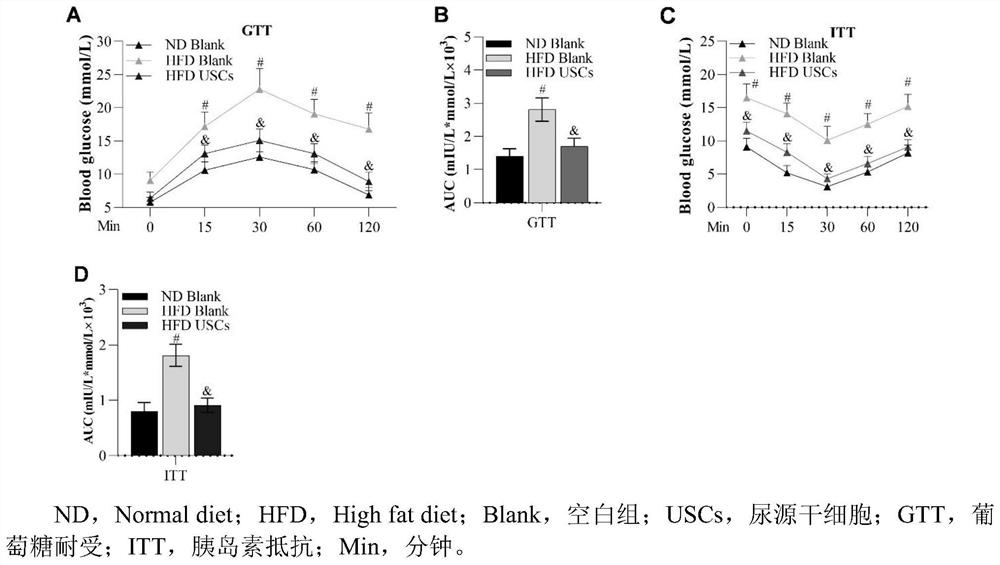
[0036] Example 2: USCs can reduce glucose tolerance and insulin resistance in HFD mice
[0037] 2.1 Experimental method:
[0038] 2.1.1 Glucose tolerance test (GTT) changes in mice detected by blood glucose meter
[0039] After tail vein injection of USCs and HFD feeding for 8w, fasting for 16h. At 0, 15, 30, 60 and 120 minutes after the intraperitoneal injection of glucose solution (2 g / kg body weight), blood was taken from the tail of the blood glucose meter to measure the blood glucose concentration.
[0040] 2.1.2 Blood glucose meter detects changes in mice (insulin tolerance test, ITT)
[0041] After tail vein injection of USCs and HFD feeding for 8w, fasting for 16h. Insulin solution (0.75U / kg body weight) was injected intraperitoneally, and at 0, 15, 30, 60 and 120 minutes after that, blood was taken from the tail of the blood glucose meter to measure the blood glucose concentration.
[0042] 2.2 Experimental results:
[0043] Experiments in mice showed that USCs c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com