Carbonic ester electrolyte additive and application thereof
A technology of electrolyte additives and carbonates, applied in the directions of organic electrolytes, non-aqueous electrolytes, non-aqueous electrolyte batteries, etc., can solve the problems of complicated operation, limited practical application, high cost, etc., and achieves a simple formula and is conducive to large batches. Production, the effect of improving cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) The reference electrolyte 1M LiPF 6 EMC / FEC (7 / 3, volume ratio) is transferred to a glass bottle, and then the additive 2-fluorophenylsulfur pentafluoride of the present invention that accounts for 5wt.% of the reference electrolyte is added in the glass bottle, and it is stirred overnight to form a uniform liquid electrolyte.
[0039] 2) Take the electrolyte in 1), and then assemble Li / / Cu half cells and Li / / Li symmetrical cells as examples.
[0040] At the same time, as a comparison, a lithium metal battery assembled with a reference electrolyte without additives was used as a comparative example, and other operations were the same as above.
[0041] Test results such as figure 1 As shown, there are a large number of needle-like lithium dendrites and loose and porous lithium metal in the comparative example, indicating that the lithium deposition is not uniform under the electrolyte system in the comparative example. In the electrolyte of the embodiment, the su...
Embodiment 2
[0044] 1) The reference electrolyte 1M LiPF 6 EMC / FEC (7 / 3, volume ratio) is transferred to the glass bottle, and then the additive 4-fluorophenyl sulfur pentafluoride of the present invention accounting for 5wt.% of the reference electrolyte is added in the glass bottle, and it is stirred overnight to form a uniform liquid electrolyte.
[0045] 2) Take the electrolyte in 1), and then assemble a Li / / LCO full battery as an example.
[0046] At the same time, as a comparison, a Li / / LCO full battery assembled with a reference electrolyte without additives was used as a comparative example, and other operations were the same as above.
[0047] Test results such as image 3 As shown, under the condition of current density of 0.2C, the first-cycle coulombic efficiency of the Li / / LCO full battery of the embodiment is 96.38%, and the first-cycle discharge capacity is 210.8mAh g -1 , far superior to the comparative example.
Embodiment 3
[0049] 1) The reference electrolyte 1M LiPF 6 EMC / FEC (7 / 3, volume ratio) is transferred to a glass bottle, and then the additive 2-fluorophenylsulfur pentafluoride of the present invention that accounts for 1wt.% of the reference electrolyte is added in the glass bottle, and it is stirred overnight to form a uniform liquid electrolyte.
[0050] 2) Take the electrolyte in 1), and then assemble Li / / Cu half cells and Li / / Li symmetrical cells as examples.
[0051] At the same time, as a comparison, a lithium metal battery assembled with a reference electrolyte without additives was used as a comparative example, and other operations were the same as above.
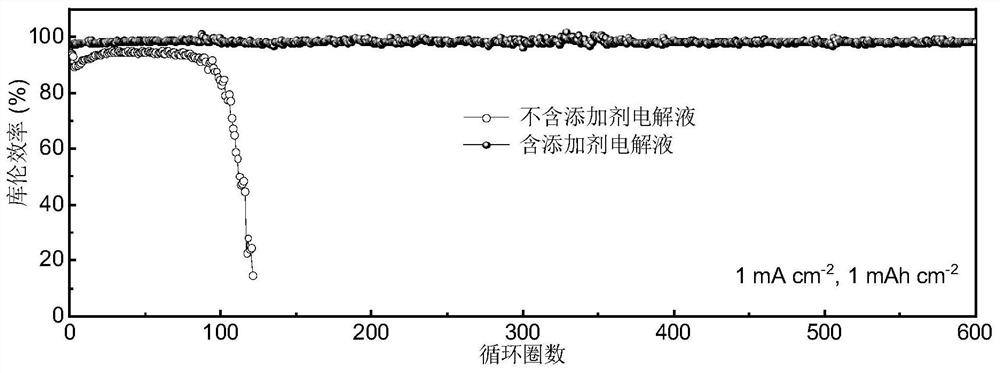
[0052] Test result: at 1mAcm -2 and 1mAh cm -2 Under these conditions, the average coulombic efficiency of the Li / / Cu battery of the embodiment after 300 cycles is 96.23%, which is far better than that of the comparative example. at 1 mA cm -2 and 1mAh cm -2 Under the conditions, the Li / / Li symmetric battery of the emb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com