1, 2-benzothiazine heterocyclic derivative as well as preparation method and application thereof
A technology for benzothiazine and derivatives, which is applied in the field of 1,2-benzothiazine heterocyclic derivatives and their preparation, and achieves the effects of improving binding capacity, increasing strength, and improving antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
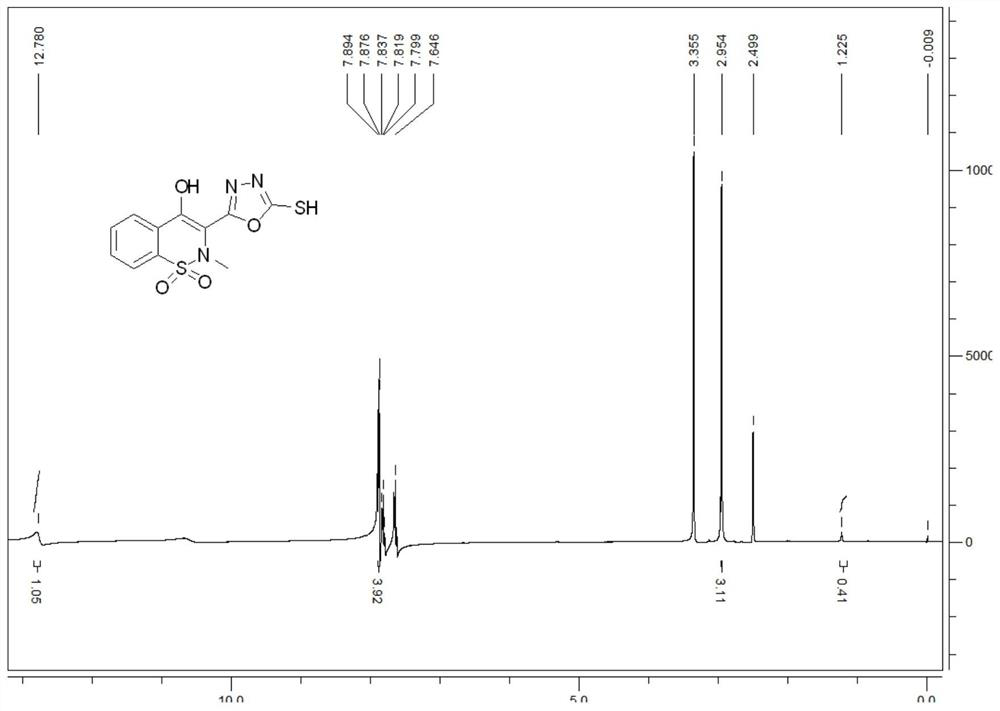
[0039] 26.880g 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylic acid methyl ester-1,1-dioxide, 18mL80wt.% hydrazine hydrate solution, 110mL absolute ethanol Add it into a 250mL three-neck flask, heat and reflux for 12 hours to obtain a yellow clear solution; distill off the solvent under reduced pressure to obtain a light yellow viscous substance, then add 40mL of absolute ethanol, heat to dissolve completely, cool naturally, filter, Collect the filter cake and blow dry at 50°C to obtain a yellow solid 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxhydrazide-1,1-dioxide 23.044 g, the yield is 85.73%.
[0040] Weigh 6g (0.087mol) of potassium hydroxide and dissolve it in 150mL of absolute ethanol. After completely dissolving, add 15g (0.055mol) of 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3- Forformylhydrazide-1,1-dioxide, cool down to about 0°C in an ice bath, control the temperature at 0-5°C, add 6mL of carbon disulfide with a concentration of 1.26g / ml dropwise with a con...
Embodiment 2
[0042] Add 40.064g of 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylic acid methyl ester-1,1-dioxide, 28mL of 80wt.% hydrazine hydrate solution, and 160mL of methanol to In a 250mL three-neck flask, heat and reflux for 12 hours to obtain a yellow clear solution; distill off the solvent under reduced pressure to obtain a light yellow viscous substance, then add 60mL of absolute ethanol, and heat to completely dissolve, naturally cool, filter, and collect the filtered cake, and air-dried at 50°C to obtain 33.714g of yellow solid 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxhydrazide-1,1-dioxide, The yield is 84.15%.
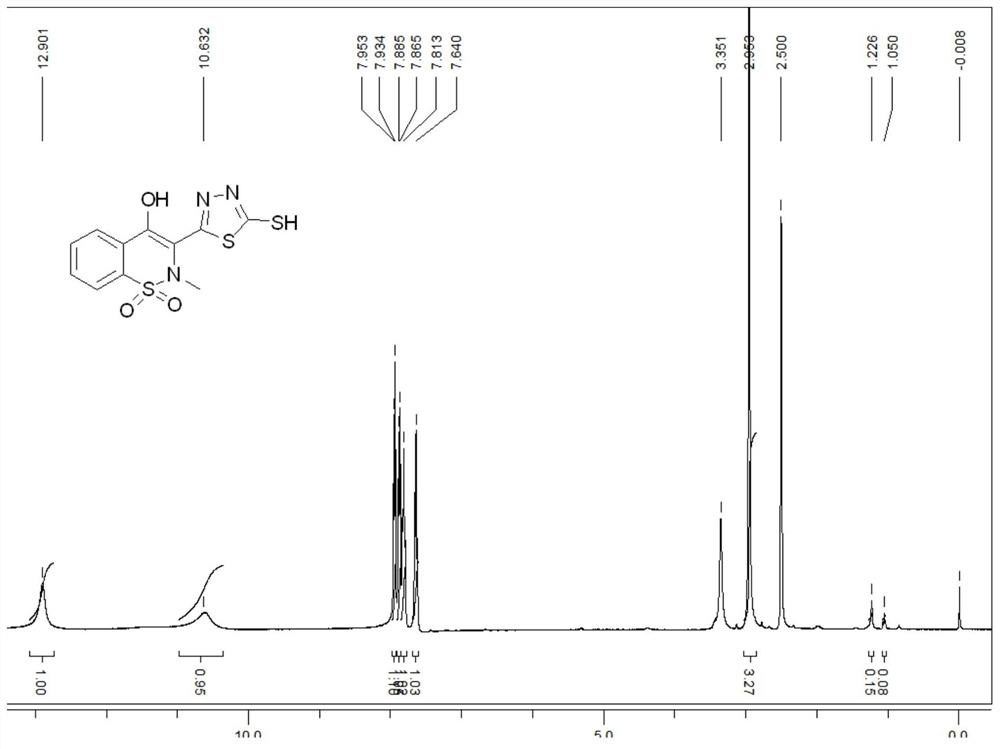
[0043] Take 10g (0.146mol) of potassium hydroxide and dissolve it in 135mL of absolute ethanol. After completely dissolving, add 26.9g (0.1mol) of 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3- Formohydrazide-1,1-dioxide, cool down to about 0°C in an ice bath, control the temperature at 0-5°C, slowly add 9mL of carbon disulfide with a concentration of 1.26g / ml ...
Embodiment 3
[0046] 26.880g 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylic acid methyl ester-1,1-dioxide, 25mL80wt.% hydrazine hydrate solution, 150mL n-propanol Add it into a 250mL three-neck flask, heat and reflux for 12 hours to obtain a yellow clear solution; distill off the solvent under reduced pressure to obtain a light yellow viscous substance, then add 50mL of n-propanol, heat to dissolve completely, cool naturally, filter, Collect the filter cake and blow dry at 50°C to obtain a yellow solid 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxhydrazide-1,1-dioxide 23.453 g, the yield is 87.25%.
[0047] Weigh 6g (0.087mol) of potassium hydroxide and dissolve it in 145mL of n-propanol. After completely dissolving, add 15g (0.055mol) of 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3- Formohydrazide-1,1-dioxide, cool down to about 0°C in an ice bath, control the temperature at 0-5°C, add 9mL of carbon disulfide with a concentration of 1.26g / ml dropwise through a constant pressure drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com