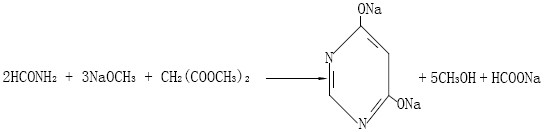
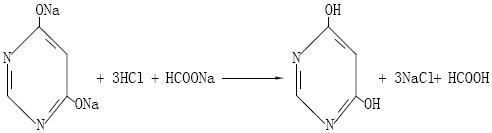
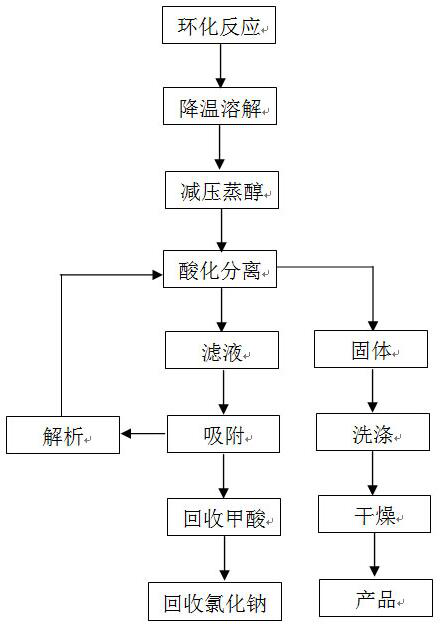
A method for preparing 4,6-dihydroxypyrimidine
A technology of dihydroxypyrimidine and hydroxypyrimidine disodium salt, which is applied in the field of 4,6-dihydroxypyrimidine synthesis, can solve the problems that 4,6-dihydroxypyrimidine cannot be recycled, difficult wastewater treatment, solid waste of activated carbon, etc. To achieve the effect of avoiding solid waste of activated carbon, solving difficult to handle, and reducing the content of organic matter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 12,600kg of methanol solution containing 30%wt sodium methoxide to a 20,000L reactor, then add 2,172kg of formamide, raise the temperature to 65°C, then start to drop 2,610kg of dimethyl malonate, and control the reaction temperature at 60°C, After the dropwise addition, keep warm for 5 hours;
[0040] (2) Cool down to 30°C, add 10080kg of water, and stir for 1 hour to dissolve 4,6-dihydroxypyrimidine disodium salt;
[0041] (3) Continuously add the fully dissolved reaction solution into the alcohol distillation tower, recover anhydrous methanol from the top of the tower, and continuously discharge the solution containing 4,6-dihydroxypyrimidine disodium salt from the bottom of the tower;
[0042] (4) Add 30%wt hydrochloric acid to the solution containing 4,6-dihydroxypyrimidine disodium salt discharged from the tower kettle for acidification. Dihydroxypyrimidine, centrifuged to obtain 4,6-dihydroxypyrimidine solid wet material and sodium chloride acidification...
Embodiment 2
[0049] (1) Add 12,600kg of methanol solution containing 30%wt sodium methoxide to a 20,000L reactor, then add 2,175kg of formamide, raise the temperature to 65°C, then start adding 2,620kg of dimethyl malonate dropwise, and control the reaction temperature at 65°C. After the dropwise addition, keep warm for 4 hours;
[0050] (2) Cool down to 35°C, add 9000kg of water, and stir for 2 hours to dissolve 4,6-dihydroxypyrimidine disodium salt;
[0051] (3) Continuously add the fully dissolved reaction solution into the alcohol distillation tower, recover anhydrous methanol from the top of the tower, and continuously discharge the solution containing 4,6-dihydroxypyrimidine disodium salt from the bottom of the tower;
[0052] (4) Add 20%wt hydrochloric acid to the solution containing 4,6-dihydroxypyrimidine disodium salt discharged from the tower kettle for acidification. Dihydroxypyrimidine, centrifuged to obtain 4,6-dihydroxypyrimidine solid wet material and sodium chloride acidifi...
Embodiment 3
[0059] (1) Add 12,600kg of methanol solution containing 30%wt sodium methoxide to a 20,000L reactor, then add 2,175kg of formamide, raise the temperature to 65°C, then start adding 2,626kg of dimethyl malonate dropwise, and control the reaction temperature at 70°C. After the dropwise addition, the insulation was kept for 4.5 hours;
[0060] (2) Cool down to 33°C, add 8000kg of water, and stir for 0.5 hours to dissolve 4,6-dihydroxypyrimidine disodium salt;
[0061] (3) Continuously add the fully dissolved reaction solution into the alcohol distillation tower, recover anhydrous methanol from the top of the tower, and continuously discharge the solution containing 4,6-dihydroxypyrimidine disodium salt from the bottom of the tower;
[0062] (4) Add 28%wt hydrochloric acid to the solution containing 4,6-dihydroxypyrimidine disodium salt for acidification, the acidification temperature is 40°C, acidify to pH 3-4, precipitate 4,6-dihydroxypyrimidine, centrifuge Filter to get 4,6-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com