5-aryl nicotinamide LSD1/HDAC double-target inhibitor and preparation method and application thereof
A technology of aryl nicotinamide and reaction, which is applied in the field of medicinal chemistry, achieves good selectivity, is conducive to popularization and application, and has the effect of strong anti-tumor activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
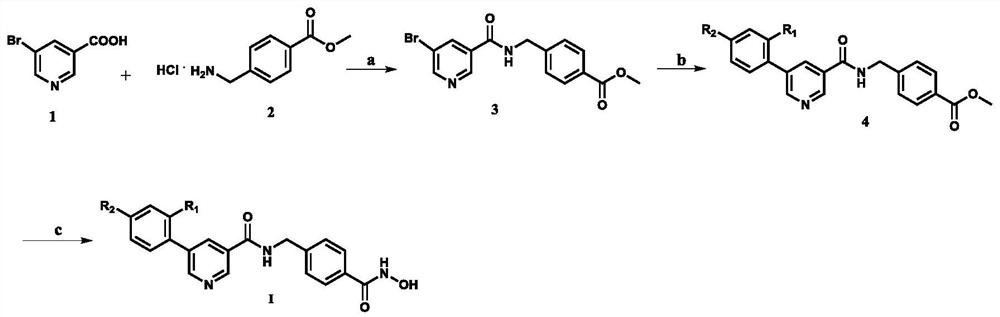
[0021] Example 1 Synthesis of 2-((5-nicotinamide) methyl)methyl benzoate (3)
[0022]
[0023] Add compound 1 (404.02mg, 2.0mmol), compound 2 (443.63 mg, 2.2mmol), HBTU (834.33mg, 2.2mmol), N 2 Protected, dissolved with anhydrous DMF (8mL), added N,N-diisopropylethylamine (568.66mg, 4.4mmol), after addition, stirred at room temperature for 1.5h, then added water and ethyl acetate to the reaction system Extract, combine the ethyl acetate layers, wash with water and saturated brine respectively, and dry over anhydrous sodium sulfate. After drying, filter, the filtrate is concentrated under reduced pressure, and the concentrate is separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 2:1) Compound 3 (434.4mg), white solid, yield: 62.2%, Mp: 160-161℃. 1 H NMR (400MHz, DMSO-d 6 )δ9.43(t, 1H, J=6.0Hz), 9.04(d, 1H, J=2.0Hz), 8.89(d, 1H, J=2.0Hz), 8.49(t, 1H, J=2.0Hz) ,7.95(d,2H,J=8.0Hz), 7.49(d,2H,J=8.4Hz),4.59(d,2H,J=6.0Hz),3.85(s,3H). 1...
Embodiment 2
[0024] Example 2 Synthesis of methyl 4-((5-(2-hydroxyphenyl)nicotinamide)methyl)benzoate (4a)
[0025] In a 50mL two-necked flask, add compound 3 (500.0mg, 1.4mmol), toluene (5mL), ethanol (5mL), H 2 O (1.3 mL), K 2 CO 3 (359.3mg, 2.6mmol), Pd(PPh 3 ) 4 (162.0mg, 0.14 mmol) and 2-hydroxyphenylboronic acid (235.0mg, 1.7mmol), under nitrogen protection, stirred and heated at 92°C for 3 hours, after the reaction was completed, cooled to room temperature, extracted the reaction system with water and ethyl acetate, combined The ethyl acetate layer was washed with water and saturated brine respectively, and dried over anhydrous sodium sulfate. After drying, it was filtered with suction, and the filtrate was concentrated under reduced pressure. The concentrate was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 2: 1), to obtain compound 4a (279.0mg), yield: 55.0%, white solid, Mp: 205-206℃. 1 H NMR (400MHz, DMSO-d 6 )δ9.89(s, 1H), 9....
Embodiment 3
[0026] Example 3 Synthesis of methyl 4-((5-(4-cyanophenylnicotinic acid amide))methyl)benzoate (4b)
[0027] According to the method of Example 2, replace 2-hydroxyphenylboronic acid with compound 4-cyanophenylboronic acid (249.8mg, 1.7mmol) to obtain target compound 4b (295.3mg), white solid, yield: 56.8%, Mp: 181 - 182°C. 1 H NMR (400MHz, DMSO-d 6 )δ9.46(t, 1H, J=6.0Hz), 9.14(d, 1H, J=2.0Hz), 9.11(d, 1H, J=2.0Hz), 8.61(t, 1H, J=2.0Hz) ,8.06(d,2H,J=8.4Hz), 8.02(d,2H,J=8.4Hz),7.95(d,2H,J=8.4Hz),7.52(d,2H,J=8.0Hz),4.64 (d, 2H, J=6.0Hz), 3.85(s, 3H). 13 C NMR (101MHz, DMSO-d 6 )δ166.56, 165.08, 150.72, 149.06, 145.27, 141.42, 133.89, 133.71, 133.52, 130.09, 129.79, 128.72, 128.48, 127.96, 119.13, 111.61, 52.566. 22 h 17 N 3 NaO 3 [M+Na] + :394.1162,Found:394.1165.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com