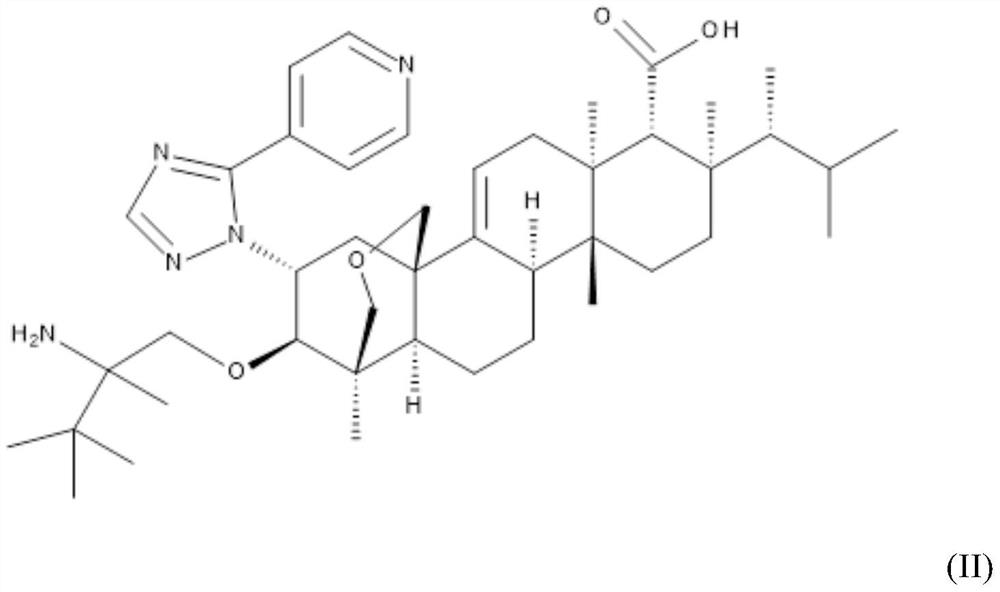
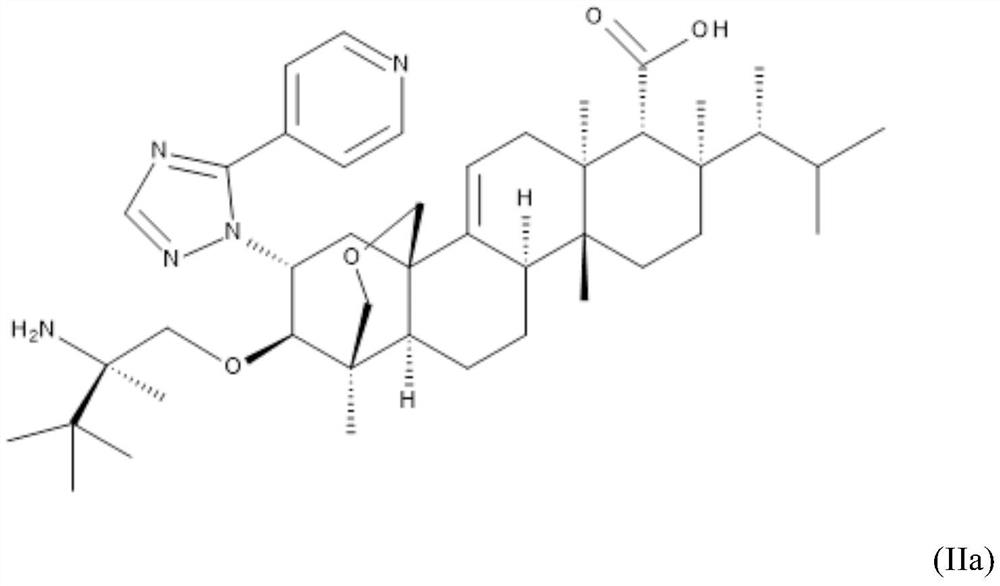
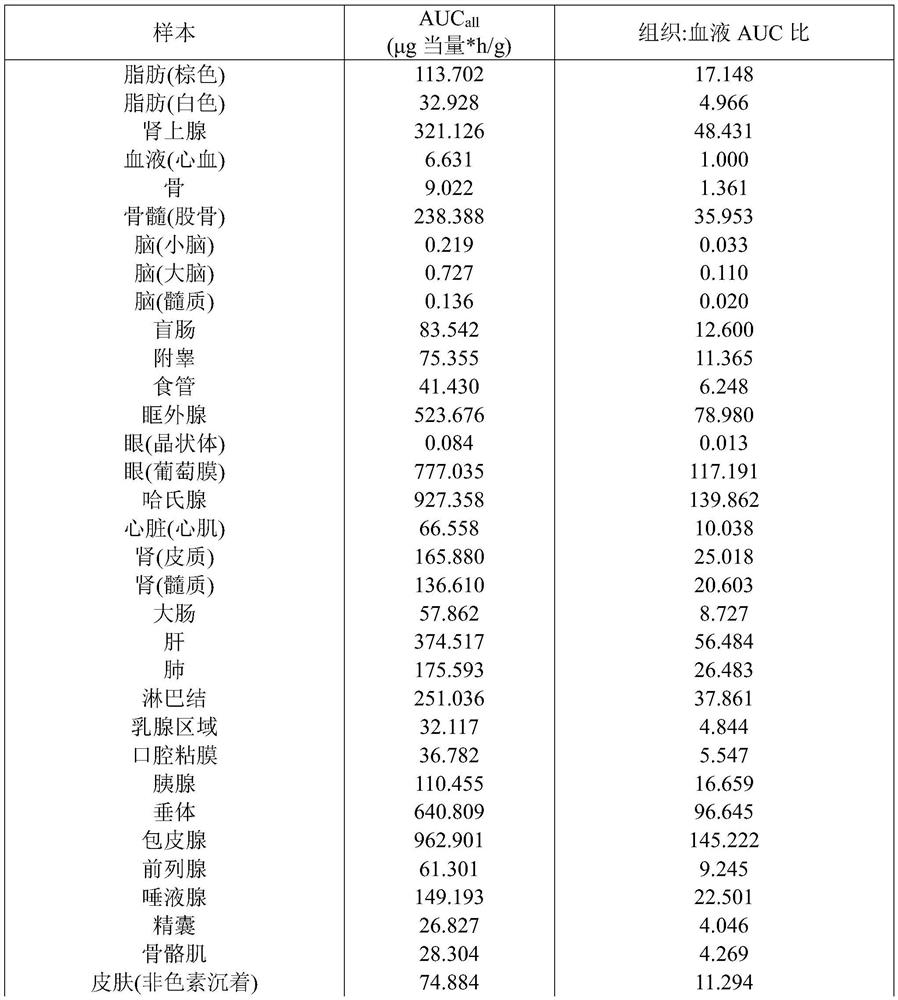
Triterpenoid antifungals for the treatment of fungal osteo-articular infections
A fungal, osteoarticular technology, used in the treatment of osteofungal infections and related connective tissue fungal infections, fungal infections, and can solve problems such as limited bone penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0116] Evaluation of Clinical Efficacy of SCY-078 Treatment of Osteogenic Fungi infection
[0117] This example shows the treatment process of patients with two candideline discitis, they participated in the "Assessment of the Efficacy and Safety of SCY-078 in the refractory or non-tolerated fungal disease (FURI) patients NCT03059992) (CLINICALTRIALS.GOV identifier NCT03059992).
[0118] OBJECTIVE: The study of the study is to assess the efficacy of oral administration of SCY-078 treatment to approved antifungal agents or non-tolerant fungal infections.
[0119] Research Design: This is a multi-center, open label, non-comparison, single arm research, aimed at assessing SCY-078 (as citrate application) in affocacy and / or severe candida disease in patients with records ≥ 18 years old Male and female subjects in the effects, safety and pharmacokinetics (PK) of the ethnoplastin disease, which is refractory or non-tolerant or description of standard care (SOC) antifungal treatment or...
example A
[0122] Example A: A 50-year-old male, diagnosed as acute myeloid leukemia (AML), before receiving the same kind of foreign stem cell transplantation (SCT) and graft anti-main disease (GVHD), suffering from L4 / L5 white candida inflammation. Five months ago, the patient had two white candida primer alpacaramia, and fungal blood contained four days and 7 days respectively. The patient participated in FURI studies and was treated with an oral Ai Ruifen (750 mg BID for 2 days, then 750 mg QD daily). After L4 / L5, the backpack vertebrae fusion (PLIF). At the time of assessment, the patient has been used in oral Ai Ruofen. 277 days. Due to pain relief, refundlacing and activity improvement, the assessment of patient responses has been rated as improved. Possible research drug-related adverse events is diarrhea, gastrointestinal air deflation and nausea.
example B
[0123] Example B: A 58-year-old male, bladder cancer is recurrent after retreating of radical bladder regeneration in the ileal catheter, suffering from L5 / S1 tropical candida. Three months ago, he had a tropical candida antifungalmia. The patient participated in FURI studies and was treated with an oral Ai Ruifen (750 mg BID for 2 days, then 750 mg QD daily). At the time of assessment, the patient has been used in oral Ai Ruofen to treat 88 days. Due to the retardation of clinical symptoms, the assessment of patient responses has been rated to date. Possible research drug-related adverse events is diarrhea.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com