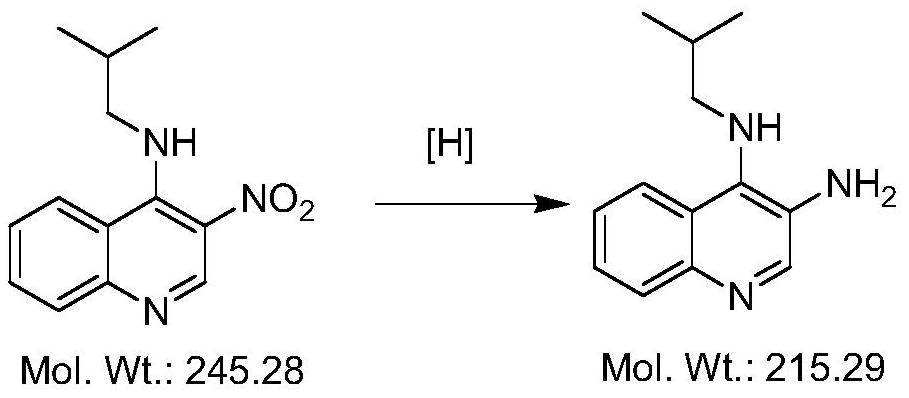
Synthesis method of imiquimod intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of imiquimod intermediate quinoline, capable of solving problems such as potential safety hazards in nitration reactions, and achieving the effects of high production efficiency, simple reaction operation, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
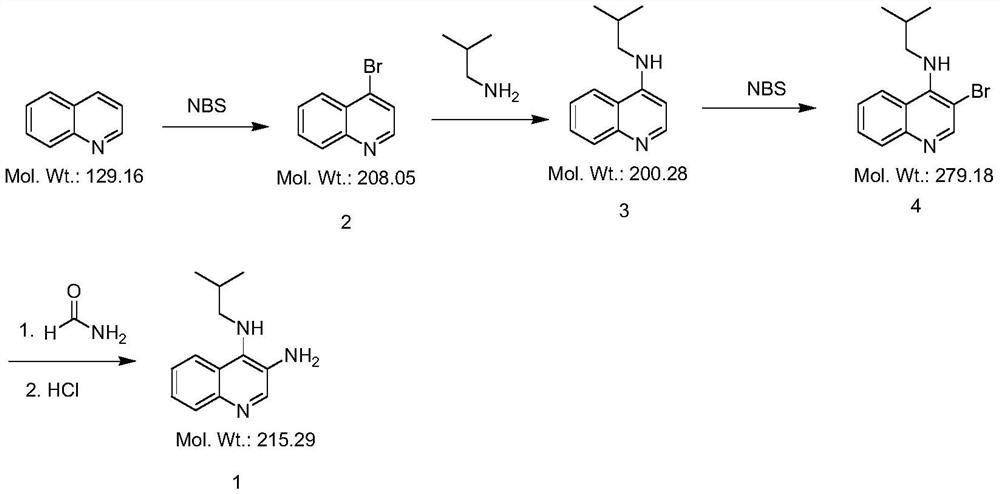
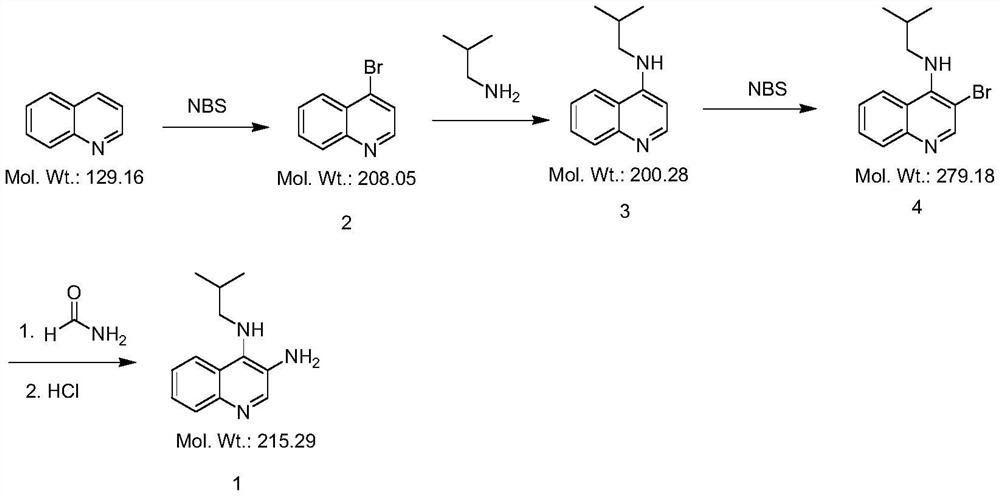
[0025] Step 1, the preparation of compound 2
[0026] In a 500ml four-neck flask, add 200ml of ethanol, 25.83g of quinoline (0.20mol), 42.72g of NBS (0.24mol) and 3.75g of copper nitrate (0.020mol) in sequence, stir at room temperature for 0.5h, then raise the temperature to 50°C for 3h . HPLC detection showed 1-2% starting material remaining. Cool down to 20°C, pour the reaction solution into 200ml of water, and stir for 2h. After suction filtration, the filter cake was stirred with 200 ml of water at room temperature for 1 h, suction filtration, and dried at 50° C. to obtain an off-white solid with a yield of 86.9%.
[0027] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.75 (d, 1H), 8.30 (dd, 1H), 8.15 (d, 1H), 7.82 (dd, 1H), 7.76 (d, 1H), 7.62 (dd, 1H).
[0028] Step 2, the preparation of compound 3
[0029] In a 250ml four-necked flask, add 150ml N-methylpyrrolidone, 20.81g compound 2 (0.10mol), 8.04g isobutylamine (0.11mol), 27.64g potassium carbonate (0.20mol), 2.49g potassiu...
Embodiment 2
[0039] Step 1, the preparation of compound 2
[0040] In a 500ml four-neck flask, add 200ml ethanol, 25.83g quinoline (0.20mol), 42.72g NBS (0.24mol) and 1.59g copper oxide (0.020mol) in sequence, stir at room temperature for 0.5h, then raise the temperature to 50°C for 3h . HPLC detection showed 1-2% starting material remaining. Cool down to 20°C, pour the reaction solution into 200ml of water, and stir for 2h. After suction filtration, the filter cake was stirred with 200 ml of water at room temperature for 1 h, suction filtration, and drying at 50°C to obtain an off-white solid with a yield of 77.3%.
[0041] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.75 (d, 1H), 8.30 (dd, 1H), 8.15 (d, 1H), 7.82 (dd, 1H), 7.76 (d, 1H), 7.62 (dd, 1H).
[0042] Step 2, the preparation of compound 3
[0043] In a 250ml four-necked flask, add 150ml N-methylpyrrolidone, 20.81g compound 2 (0.10mol), 8.04g isobutylamine (0.11mol), 8.02g sodium hydroxide (0.20mol), 2.49g potassium iodide (0.015 mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com