Kit for detecting SARS-CoV-2 coronavirus and special primer and probe thereof
A technology for SARS-CoV-2 and coronavirus, which is applied in the field of detection kits and special primers and probes for SARS-CoV-2 coronavirus, which can solve the problems of strong infectivity and achieve high detection sensitivity and application prospects Wide, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Primer and probe design
[0033] This embodiment aims to determine that primers and probes that can detect SARS-COV-2 coronavirus quickly by using fluorescent quantitative polymerase amplification technology, the inventor utilizes the surface protein S gene (SEQ ID NO: 1), according to the design principles of primers and probes, design multiple sets of primers and probe combinations, select the following groups 1 and 2 from the various combinations, and the sequence information is as follows:
[0034] Group 1:
[0035] SA-F1: 5'-TGCCTTGGTGATATTGCTGC-3' (SEQ ID NO: 2);
[0036] SA-R1: 5'-GTACCCGCTAACAGTGCAGAA-3' (SEQ ID NO: 3);
[0037] SA-probe1: 5'-FAM-CGGCCTTACTGTTTTGCCACCTTTGC-BHQ-3' (SEQ ID NO: 4);
[0038] The amplified sequences of primers SA-F1 and SA-R1 are:
[0039]5'-TGCCTTGGTGATATTGCTGCTAGAGACCTCATTTGTGCACAAAAGTTTAACGGCCTTACTGTTTTGCCACCTTTGCTCACAGATGAAATGATTGCTCAATACACTTCTGCACTG TTAGCGGGTAC-3' (SEQ ID NO: 5);
[0040] Group 2:
[0041] SA-F2...
Embodiment 2
[0048] Embodiment 2: establish the method for rapidly detecting SARS-COV-2
[0049] This embodiment adopts the primer and probe of group 1 that above-mentioned embodiment 1 obtains and establishes the method for rapid detection SARS-COV-2 coronavirus, specifically comprises the following steps:
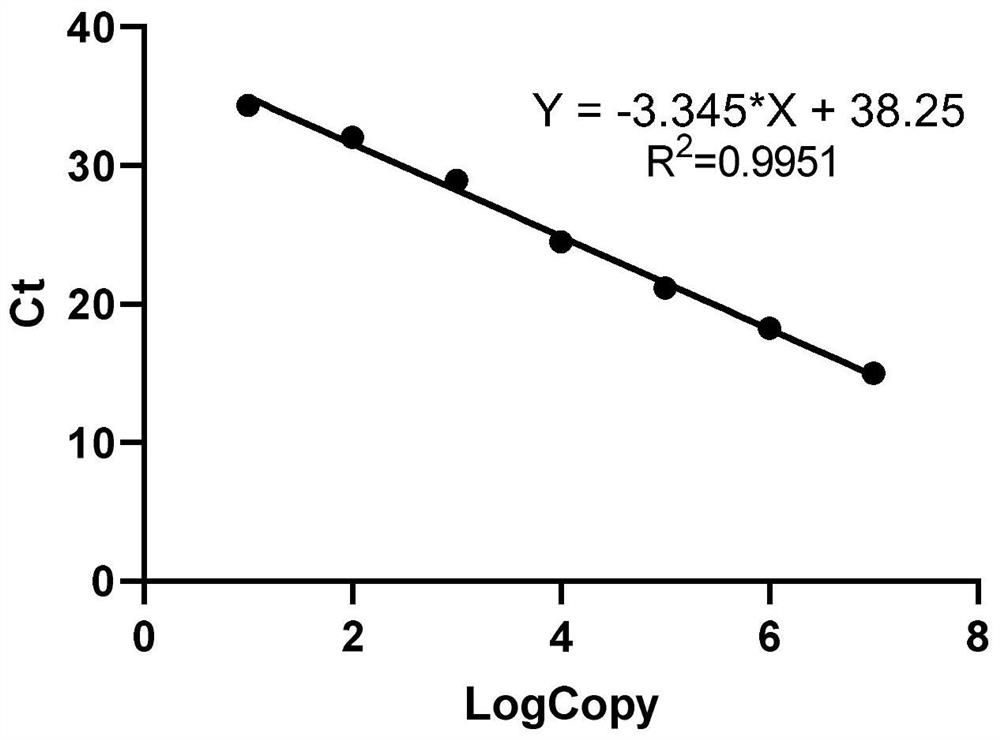
[0050] 2.1. Establish a standard curve: Dilute the positive quality control product obtained in the above-mentioned Example 1 according to a 10-fold gradient to obtain the following gradient concentration solution as a SARS-COV-2 standard substance: 1×10 7 copies / μL, 1×10 6 copies / μL, 1×10 5 copies / μL, 1×10 4 copies / μL, 1×10 3 copies / μL, 1×10 2 copies / μL, 1×10 1 copies / μL, with different concentrations of standard products as templates, under the guidance of the primers and probes shown in Group 1 (Example 1), carry out fluorescent quantitative PCR detection, wherein the system of PCR detection uses the One Step PrimeScript of TAKARA company TM RT-PCR Kit, as shown in Table 1 be...
Embodiment 3
[0069] Embodiment 3, the sensitivity of carrying out real-time fluorescent quantitative PCR detection to SARS-COV-2 coronavirus
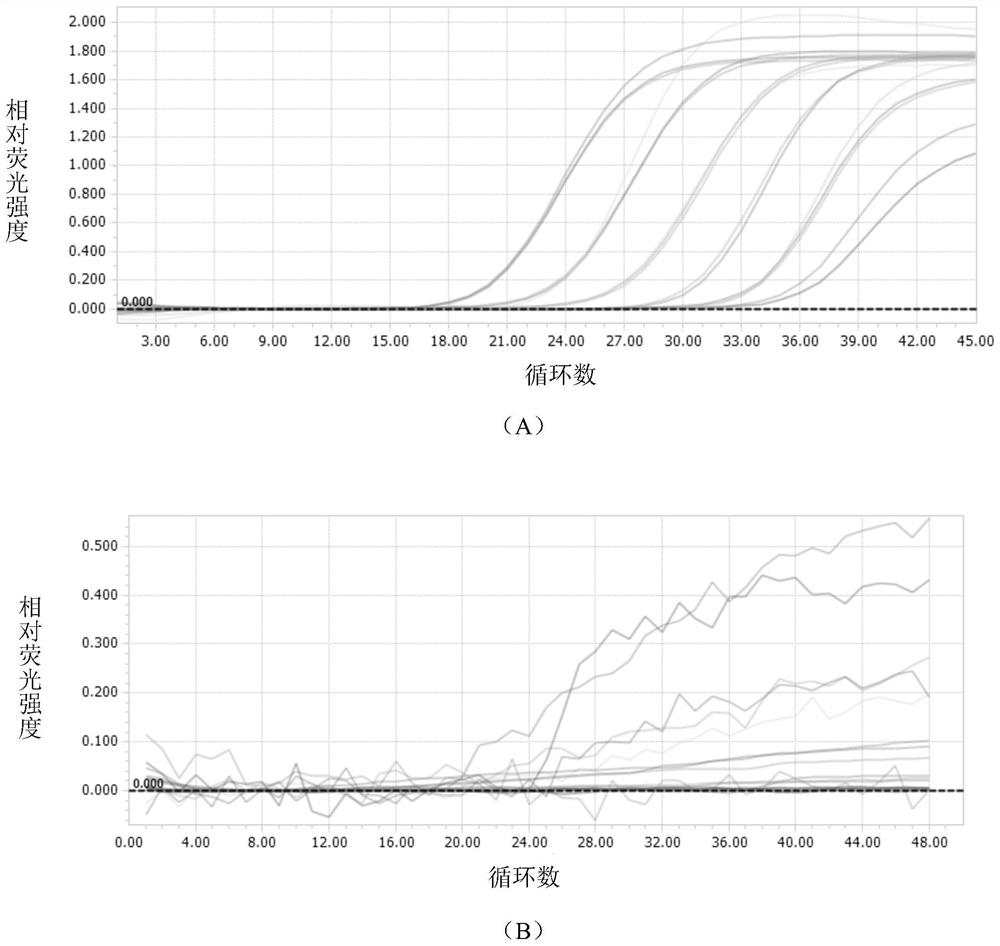
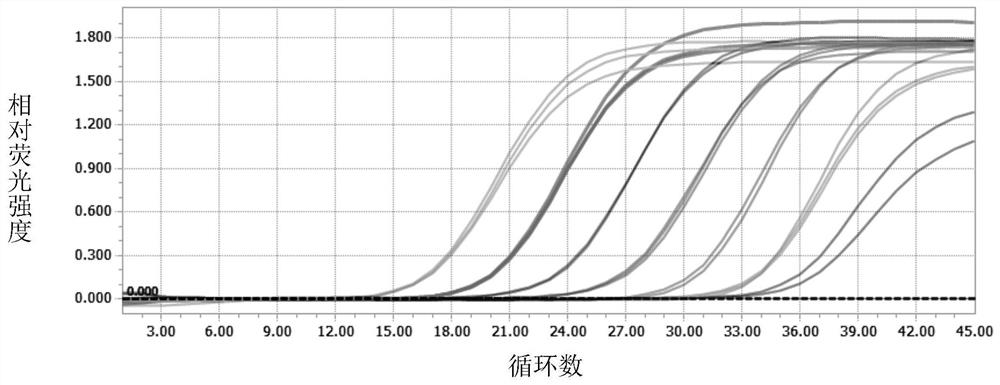
[0070] figure 1 Middle A and figure 2 Also all show the sensitivity of the detection method that the present invention carries out real-time fluorescent quantitative PCR to SARS-COV-2 coronavirus, it can be seen that the present invention carries out the detection method of real-time fluorescent quantitative PCR to SARS-COV-2 coronavirus and can detect to 1 ×10 1 copies / μl, and the amplification curve is a specific "S" curve, indicating that the primers and probes of the present invention are suitable for combining with SARS-COV-2 coronavirus RNA, and have higher sensitivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com