Novel benzodiazepine derivatives and uses thereof
A compound, heteroaryl technology, applied in the field of new benzodiazepine derivatives, can solve problems such as low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
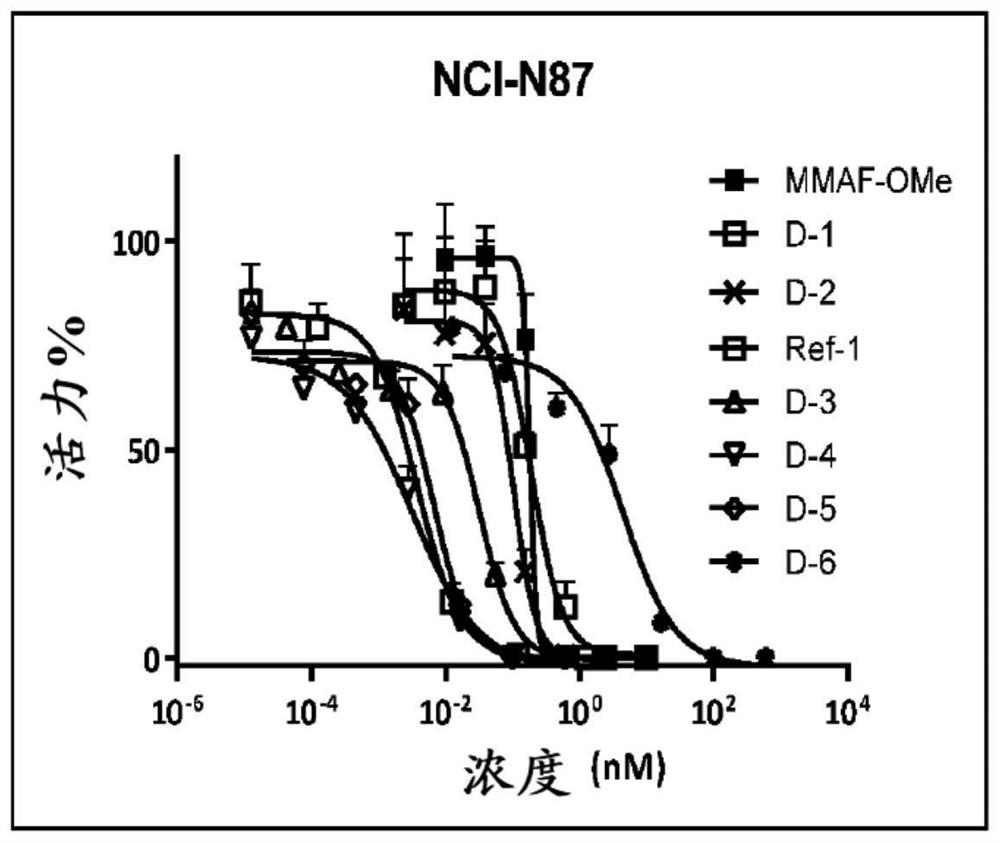
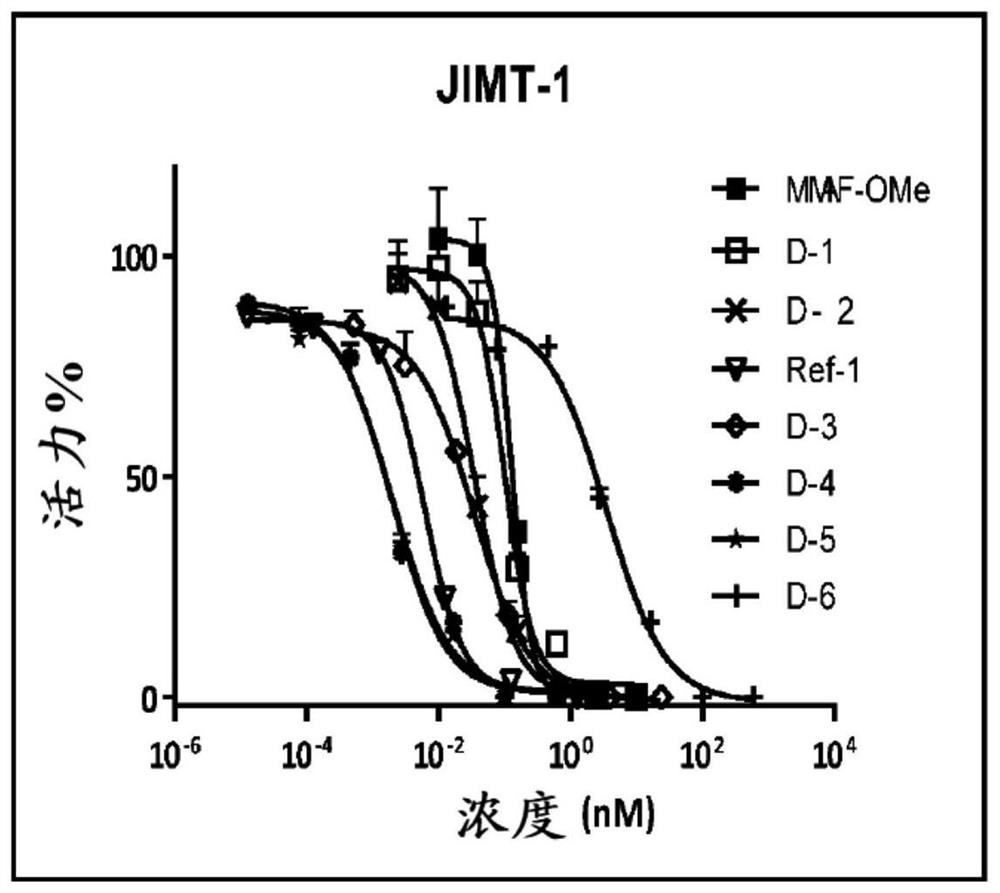
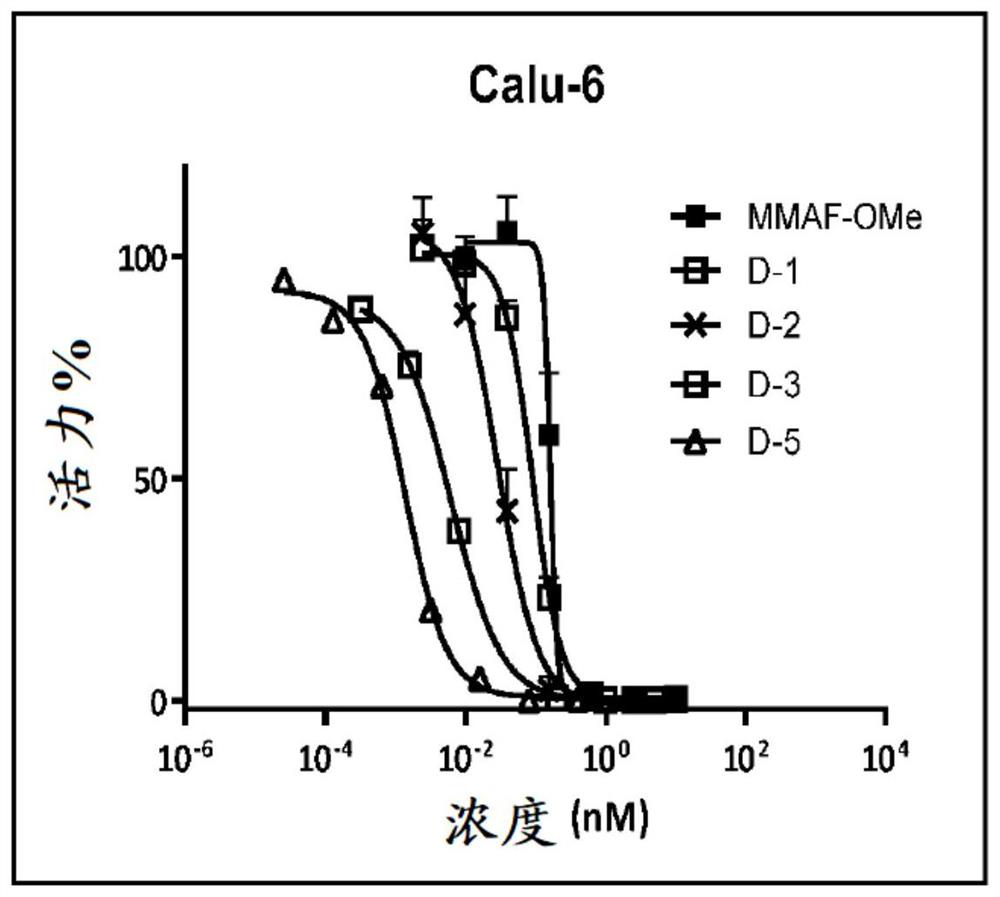
Image
Examples
Embodiment 1
[0633] Example 1: Preparation of Compound L-1
[0634]
[0635] Preparation of Compound L-1-1
[0636] at -78°C and N 2 To a solution of dimethyl 5-hydroxyisophthalate (5 g, 23.79 mmol) in anhydrous THF (300 mL) was added LAH (3.6 g, 95.15 mmol) dropwise under atmosphere. The reaction mixture was stirred at room temperature for 17 hours. After the reaction was complete, 15% NaOH solution (4 mL), H 2 O (8 mL) and EA (100 mL), then the reaction mixture was stirred for 1 h. The mixture was filtered and concentrated under reduced pressure. The residue was purified by column chromatography to obtain compound L-1-1 (3.02 g, 82%).
[0637] 1 H NMR (400 MHz, DMSO-d6) δ 6.66 (s, 1H), 6.58 (s, 2H), 4.38 (s, 4H).
[0638] Preparation of Compound L-1-2
[0639] in N 2 A solution of compound L-1-1 (2 g, 12.97 mmol) was dissolved in HBr (5.0 mL, 33% in AcOH) under atmosphere. After stirring at 60°C for 18 hours, by adding NaHCO 3 The solution (pH~8) quenched the reaction...
Embodiment 2
[0644] Embodiment 2: the preparation of compound L-2
[0645]
[0646] Preparation of compound L-2-1
[0647] at 0°C and N 2 To a solution of 3,5-pyridinedicarboxylic acid (1.0 g, 5.98 mmol) in anhydrous THF (50 mL) was added boron trifluoride tetrahydrofuran complex (30.0 mL, 30.0 mmol, 1 M THF) under atmosphere . The reaction was allowed to warm to room temperature and stirred for 18 hours. The mixture was quenched with 2N HCl until pH 2, and extracted with distilled water (20 mL) and EA (50 mL x 2). The organic layer was washed with anhydrous Na 2 SO 4 Dry, filter and concentrate under reduced pressure. The residue was purified by preparative TLC to obtain compound L-2-1 (363 mg, 48%).
[0648] 1 H NMR (400MHz, DMSO-d 6 )δ8.38 (s, 2H), 7.99 (s, 1H), 5.59 (t, J=4.0Hz, 2H), 4.61 (d, J=5.2Hz, 2H).
[0649] Preparation of Compound L-2
[0650] A mixture of L-2-1 (100 mg, 0.72 mmol) and HBr (1.5 mL, 48% in AcOH) was stirred at 120°C for 3 hours. with NaHCO ...
Embodiment 3
[0652] Embodiment 3: the preparation of compound L-3
[0653]
[0654] Preparation of Compound L-3-1
[0655] at room temperature and N 2 Under atmosphere, 4-chloropyridine-hydrochloride (1.0 g, 6.67 mmol) and diethanolamine (1.05 g, 10.00 mmol) were treated with NaOH (1.07 g, 26.67 mmol) in H 2 O (12 mL) and heated to 110 °C for 1 h using a microwave reactor. After the reaction was quenched with distilled water (18 mL) / methanol (10 mL) and extracted with EA (200 mL). The organic layer was washed with anhydrous Na 2 SO 4 Dry, filter and concentrate under reduced pressure. The residue was purified by column chromatography to obtain compound L-3-1 (160 mg, 13%).
[0656] 1 H NMR (400MHz, DMSO-d6) δ 8.10 (d, J = 5.6Hz, 2H), 6.83 (d, J = 6.0Hz, 2H), 4.91 (br s, 2H), 3.57 (s, 8H). EI-MS m / z:183(M + +1).
[0657] Preparation of Compound L-3
[0658] A mixture of compound L-3-1 (10 mg, 0.05 mmol) and HBr (2.0 mL, 48% in water) was reacted in a microwave at 150°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com