A compound with double inhibitory effects of egfr and wnt, preparation method and application thereof
A technology of inhibition and compound, applied in the direction of organic chemistry, medical preparations containing active ingredients, drug combination, etc., to achieve the effects of strong practical value, easy to achieve large-scale production, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of the compound of formula I (abbreviated as JK11):
[0041]
[0042] At room temperature, the compound of formula II (4-(4-amino-1H-pyrazol-1-yl)-1-tert-butoxycarbonyl-piperidine, 53.3 mg, 0.2 mmol) was first dissolved in DMF (5 ml), Then add potassium bicarbonate aqueous solution (3mol / L, 0.53ml, 1.6mmol) and FSO 2 N 3 (Fluorosulfonyl azide) in methyl tert-butyl ether solution (0.4mol / L, 2.2ml, 0.44mmol), the reaction system was mixed uniformly and then stirred at room temperature for 1 hour;
[0043] Finish the reaction, add 0.25ml of sodium ascorbate aqueous solution (0.5mol / L, 0.125mmol) to the reaction system to quench the reaction to obtain a reaction solution containing the compound of formula III;
[0044] Then to the reaction solution was added the compound of formula IV (N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-quinazolin-4-amine hydrochloride, 77.4 mg, 0.18mmol) and a catalyst formed by compounding 0.2ml CuSO4 aqueous solutio...
Embodiment 2
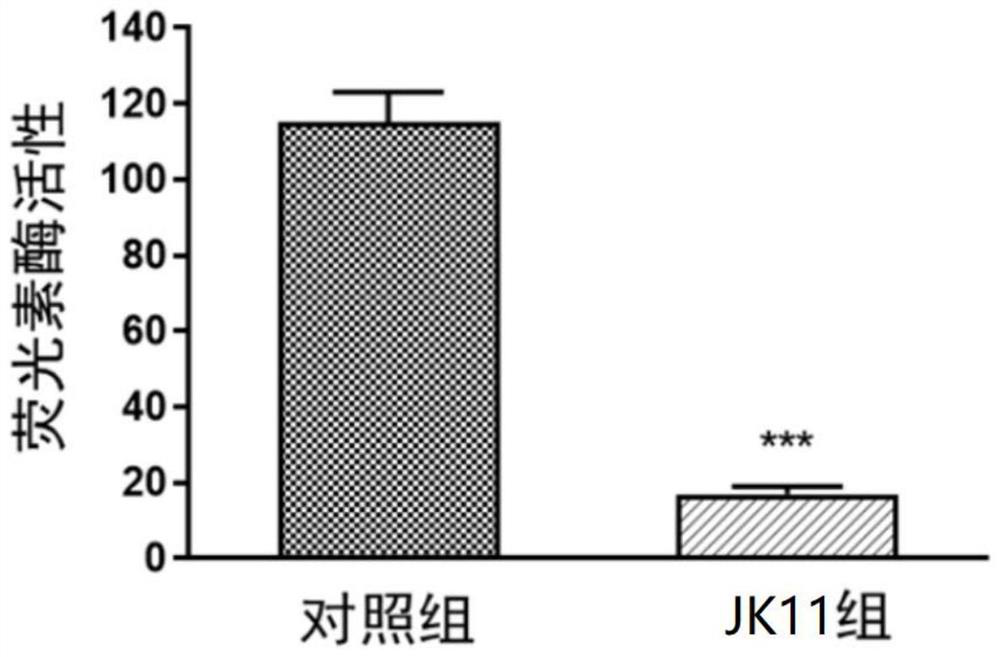
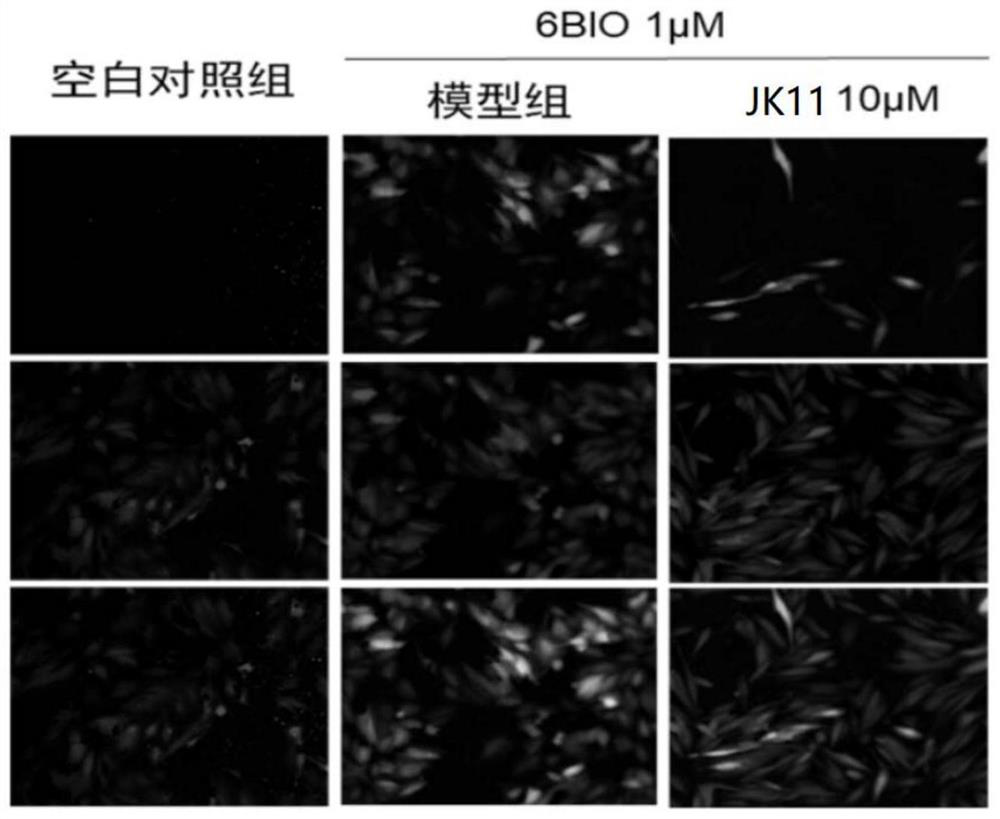
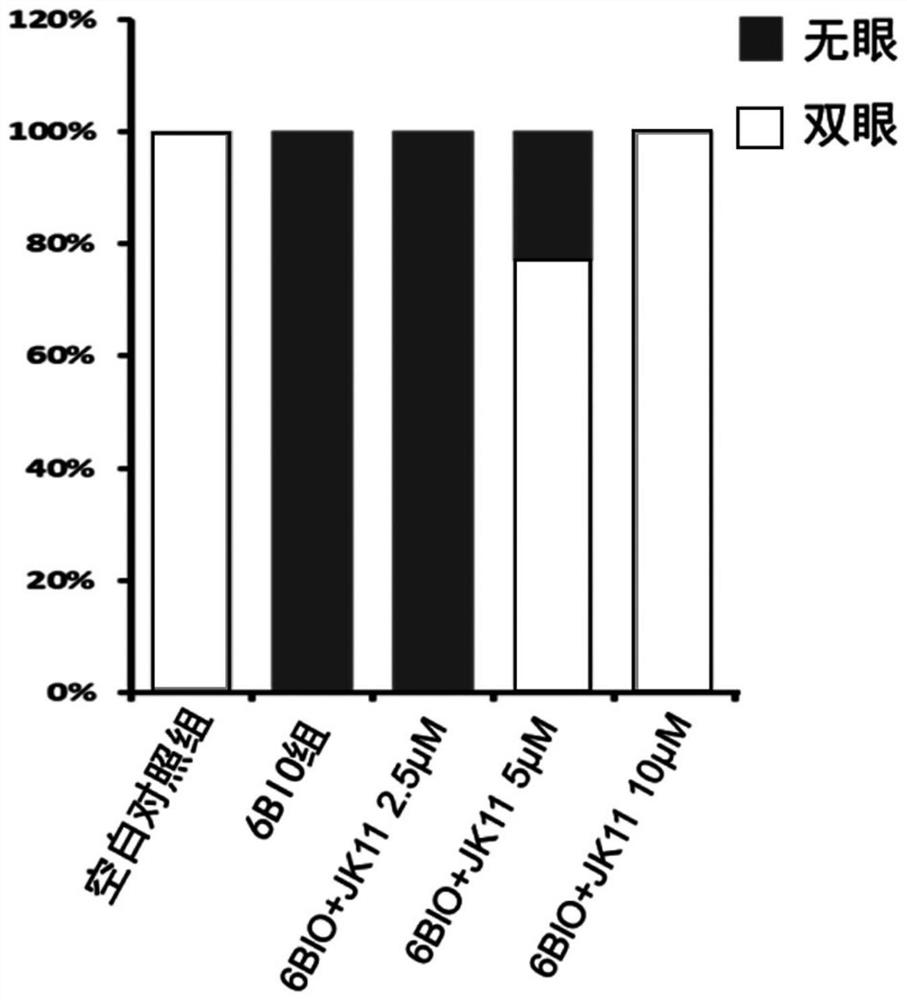
[0048] Example 2: Inhibitory effect of compound of formula I on Wnt / β-catenin pathway
[0049] Experimental materials: 293FT cells were purchased from Cell Bank of Chinese Academy of Sciences; EPT2 cells stably integrated with TGC reporter system were purchased from University of Bergen, Norway; HCT116 cells were purchased from Cell Bank of Chinese Academy of Sciences; SW480 cells were purchased from ATCC; AB line zebrafish were purchased from National Zebra Fish Resource Center.
[0050] TOP / Flash experimental procedure: 293FT cells were seeded into 96-well white plates (20,000 cells / well), and 24 hours after seeding, they were transfected with TOP / Flash plasmid and Renilla plasmid, and 6 hours later, JK11 (10 μM) was administered. After 24 hours of treatment, detection luciferase activity.
[0051] EPT2-TGC cell validation steps: EPT2 cells stably integrated with the TGC reporter system were seeded in 96-well plates and given 1 μM 6-Bronoindirubin-3'-oxime 12 hours later )...
Embodiment 3
[0057] Example 3: Inhibitory effect of compound of formula I on EGFR
[0058] Experimental materials: A549 cells were purchased from the Cell Bank of the Chinese Academy of Sciences; A549 cells were purchased from the Cell Bank of the Chinese Academy of Sciences; P-EGFR, EGFR and GAPDH antibodies were purchased from CST; the control drug Erlotinib was purchased from Sigma.
[0059] EGFR kinase assay: EGFR kinase inhibitory activity was tested using the LANCE Ultra enzyme activity assessment method. Add 2.5 μL of kinase solution to each well according to the arrangement, add 2.5 μL of reaction solution to control wells, add 2.5 μL of compound (JK11 or positive control)) solution to each well according to arrangement, add 2.5 μL of reaction solution to control wells, add 2.5 μL of reaction solution to each well according to arrangement 5μL of base solution, 10μL of Eu-anti-phospho-4E-BP1 and EDTA reaction reagents were added to each well, centrifuged for mixing, and left to equi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com