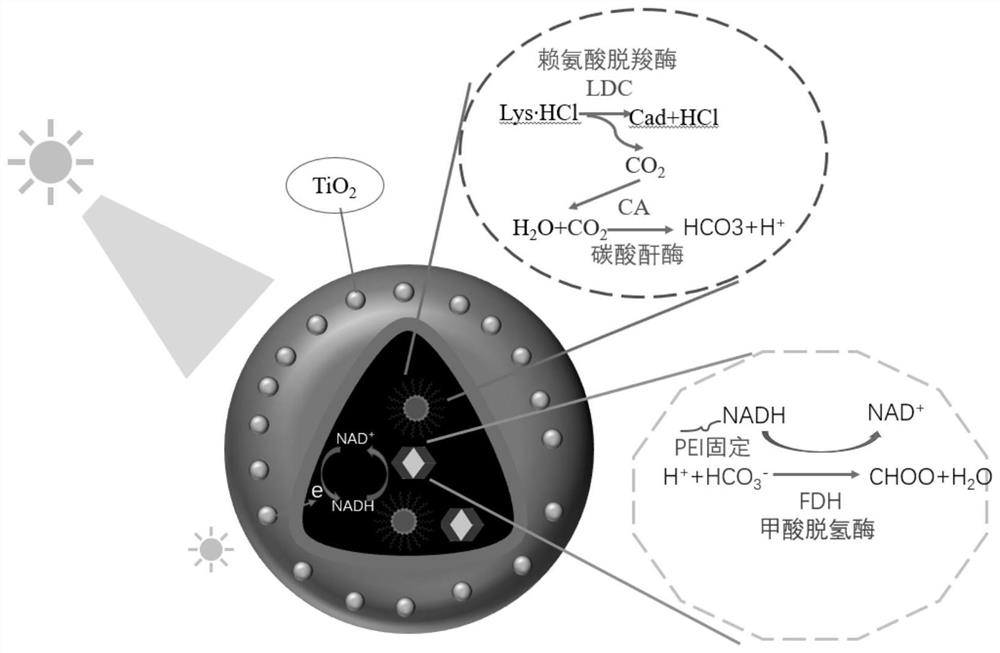
Construction method of photoactive artificial cell and application of photoactive artificial cell in synchronous green synthesis of pentamethylene diamine and formic acid
A technology of artificial cells and construction methods, applied in the field of artificial cells, can solve problems such as reduced atom economy, carbon loss, and high environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Production of pentamethylenediamine and formic acid by novel photoactive artificial cells
[0028] 1.1 Construction of Nanomaterial A
[0029] The synthesis system is 10ml, and 10mg of lysine decarboxylase (obtained from the BL21 (DE3) / pCDF-duet-CadA strain expressing E. coli-derived L-lysine decarboxylase, has been published in patent CN201810195975.2 ) and carbonic anhydrase (purchased from Shanghai Yuanye Biotechnology Co., Ltd., item number: s10157-50mg) were dissolved in deionized water, stirred at 300 rpm for 1-2 min to make the reaction solution evenly mixed, and 40 mM cobalt ions were added to increase the stirring speed Stir at 500 rpm for 10 min, and add H with a final concentration of 5 mM. 2 O 2 , stir for 30 min, centrifuge, take part of the supernatant to measure the protein concentration, wash the precipitate with deionized water 2-3 times, and use a vacuum freeze dryer to dry to obtain nanomaterial A.
[0030] Construction of Nanomaterial B ...
Embodiment 2
[0040] Example 2 Analysis method for comparison
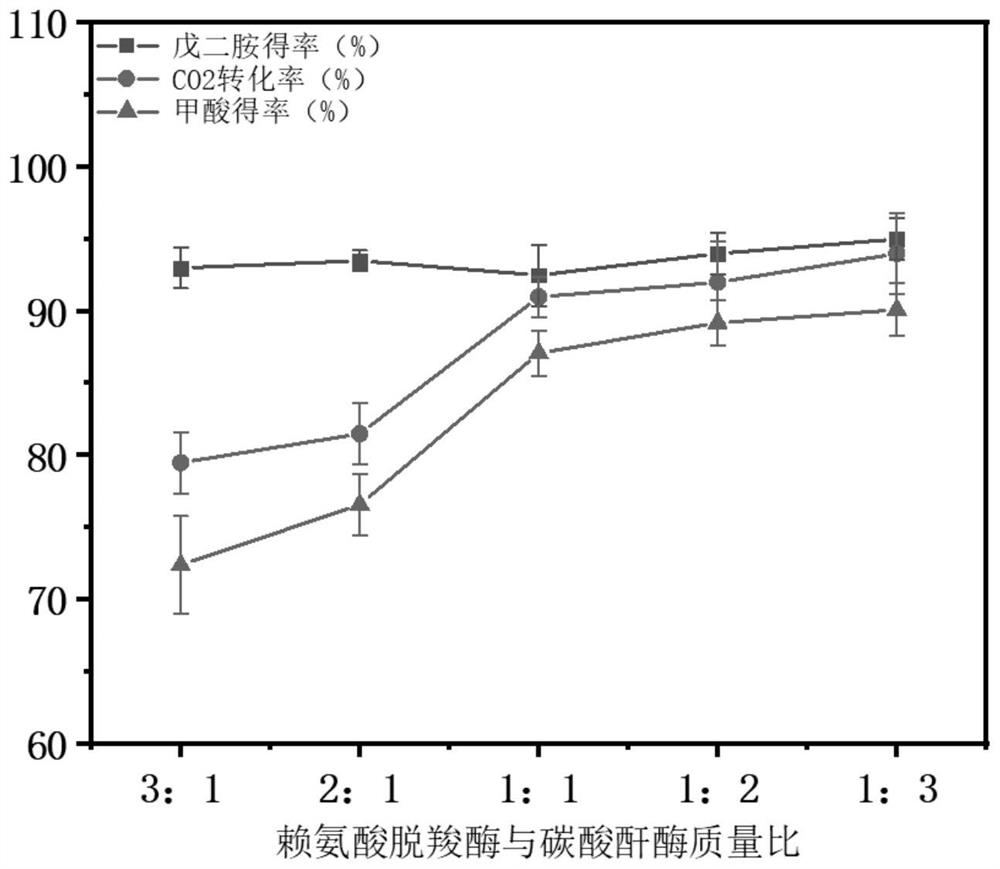
[0041] The consumption of L-lysine was detected by SBA-40E dual-channel biosensor, and the concentration of 1,5-pentanediamine was determined by Agilent 1290 liquid chromatography system and Agilent TC-C18 column (4.6×250mm). Column temperature 40±1℃, flow rate 1.0mL·min -1 ; The injection volume is 10μl; the excitation wavelength of the fluorescence detector is 350nm, and the emission wavelength is 520nm. UV detector wavelength 250nm. Formic acid concentration was measured using an Agilent 1260 liquid chromatography system and an Aminex HPX-87H column (300 × 7.88 mm). Column temperature 65±1℃, flow rate 0.6mL·min -1 ; Injection volume 10μl; UV detector wavelength 210nm. Calcium carbonate is generated by adding calcium chloride solution to the reaction solution, and the quality of calcium carbonate is weighed to detect CO. 2 conversion rate. The calculation formula is as follows:
[0042]
Embodiment 3
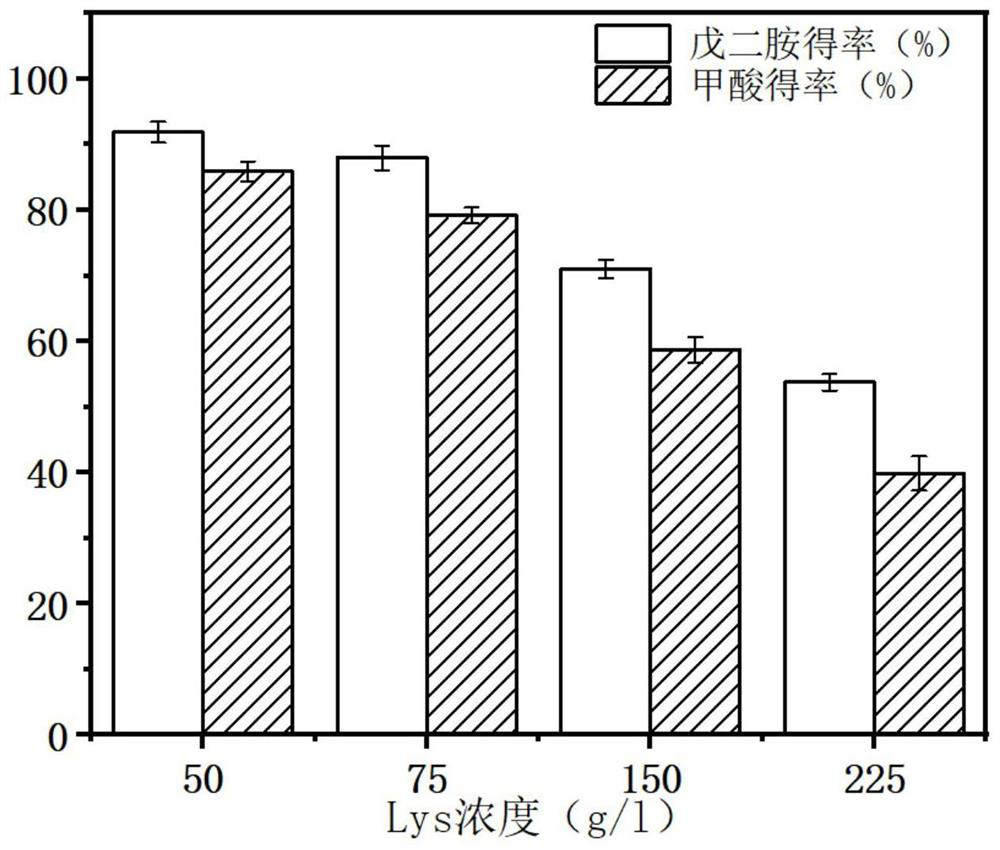
[0044] The concentration of L-lysine hydrochloride was adjusted from 50g / l to 75-225g / l, and the rest were the same as 1.6 in Example 1. The production of pentamethylenediamine and formic acid was detected by liquid chromatography, such as figure 2 shown.
[0045] from figure 2 It can be seen that when the concentration of L-lysine hydrochloride is 50g / l, the yields of pentamethylenediamine and formic acid are the highest, which are 95% and 81.5%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com