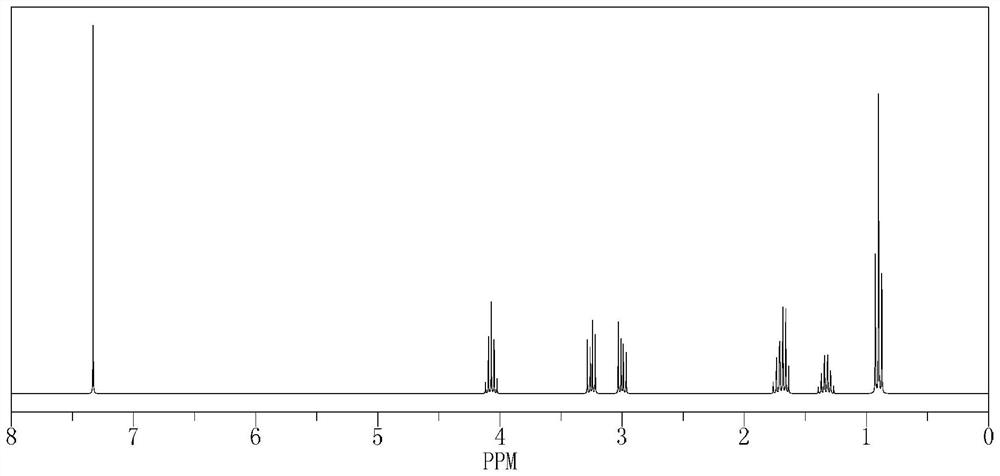
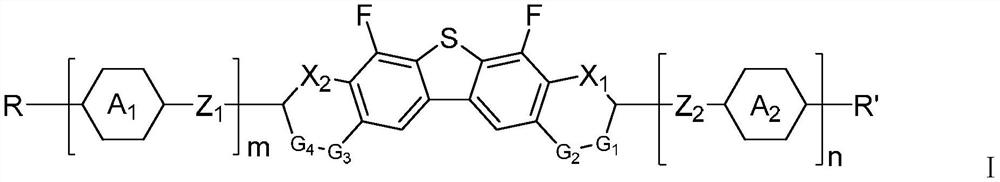
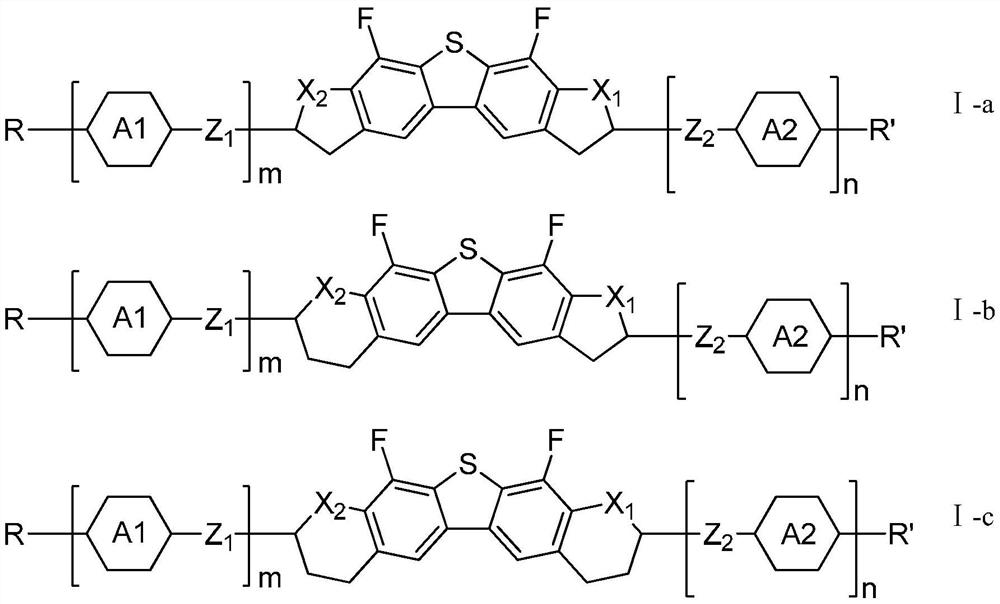
Liquid crystal compound containing dibenzothiophene and oxygen-containing heterocycles and application of liquid crystal compound
A liquid crystal compound and compound technology, applied in liquid crystal materials, chemical instruments and methods, etc., can solve the problems of poor mutual solubility and easy precipitation of liquid crystal compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] As the preparation method of the compound represented by the aforementioned formula I of the present invention, the following synthetic route is preferably adopted, and other similar structures can also be synthesized by this method.
[0062] Synthetic route 1
[0063] step 1:
[0064]
[0065] Step 2:
[0066]
[0067] Synthetic route 2
[0068] step 1:
[0069]
[0070] Step 2:
[0071]
[0072] The reactants and reagents used in the aforementioned synthetic route can all be purchased through commercial channels, and means such as the principles of such methods, operating procedures, conventional post-treatment, silica gel column, recrystallization and purification are well known to those skilled in the art. The synthesis process can be fully realized to obtain the target product.
[0073] The reactions in the aforementioned synthetic routes are all carried out in a solvent. As such a solvent, for example, a solvent selected from tetrahydrofuran, N,N-...
Embodiment 1
[0121] The preparation of the compound shown in the following formula I-a-1-1 of structural formula:
[0122]
[0123] Its preparation route is as follows:
[0124]
[0125] The specific operation process of preparation:
[0126] Step 1: Preparation of Intermediate 1
[0127] Throw 0.15mol of diisopropylamine and 0.3L of tetrahydrofuran into a 1L three-necked flask, cool down to 0°C, add 0.12mol of butyl lithium dropwise, keep warm at 0-5°C, and keep warm for half an hour; add 0.1L of 3,4-difluoro A solution of bromobenzene (0.1mol) in tetrahydrofuran was kept at -5-0°C for 1.5 hours, DMF (0.1mol) was added dropwise, and kept at -5-0°C for 2 hours. After the reaction is complete, add 500 mL of 1 mol / L dilute hydrochloric acid, let stand to separate the liquids, extract the water phase with 0.1 L×2 ethyl acetate, dry the liquid with anhydrous sodium sulfate, spin dry, dissolve 1 L of toluene and pass through the column, and evaporate the solvent to dryness. 18.8 g of t...
Embodiment 2
[0151] The preparation of the compound shown in the following formula I-a-9-1 of structural formula:
[0152]
[0153] Its preparation route is as follows:
[0154]
[0155] The specific operation process of preparation:
[0156] Step 1: Preparation of Intermediate 1
[0157] Using 1-(2-bromoethyl 1)-4-ethylbenzene and intermediate 2 of Example 1 as raw materials, refer to Step 3 of Example 1 to synthesize Intermediate 1.
[0158] Step 2: Preparation of Intermediate 2
[0159] Synthetic intermediate 2 in step 4 in reference example 1.
[0160] Step 3: Preparation of Intermediate 3
[0161] Synthetic intermediate 3 in step 10 of reference example 1.
[0162] Step 4: Preparation of Intermediate 4
[0163] Synthetic intermediate 4 in step 11 in reference example 1.
[0164] Step 5: Preparation of 1-a-9-1
[0165] The product 1-a-9-1 was synthesized with reference to step 12 in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com