Arylation or alkynylation modification method of natural amino acid-oriented polypeptide C (sp3)-H bond
A natural amino acid and modification method technology, applied in the field of post-synthesis modification of polypeptides, can solve the problems of lack of structural diversity, poor pharmacological properties, etc., and achieve a wide range of substrate applicability, high atom economy and step economy, and reaction selection sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
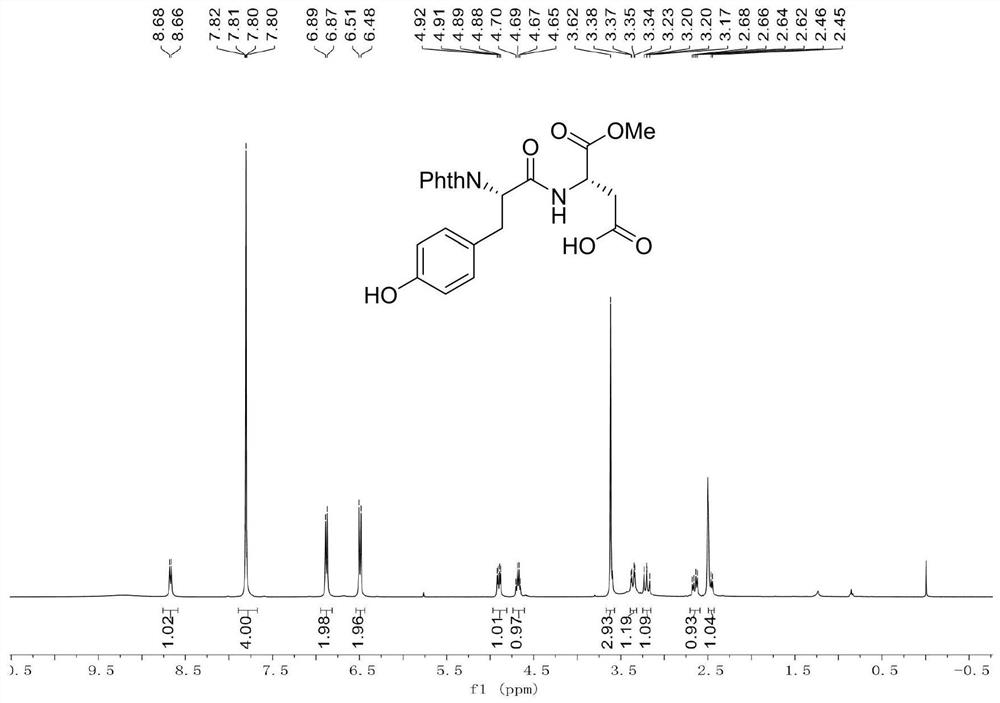
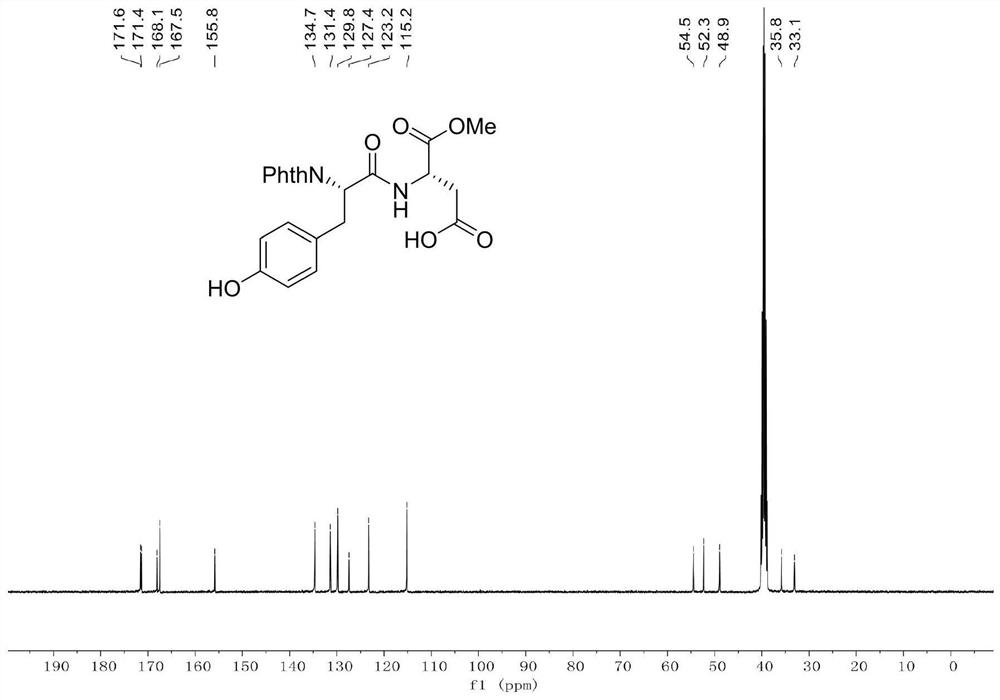
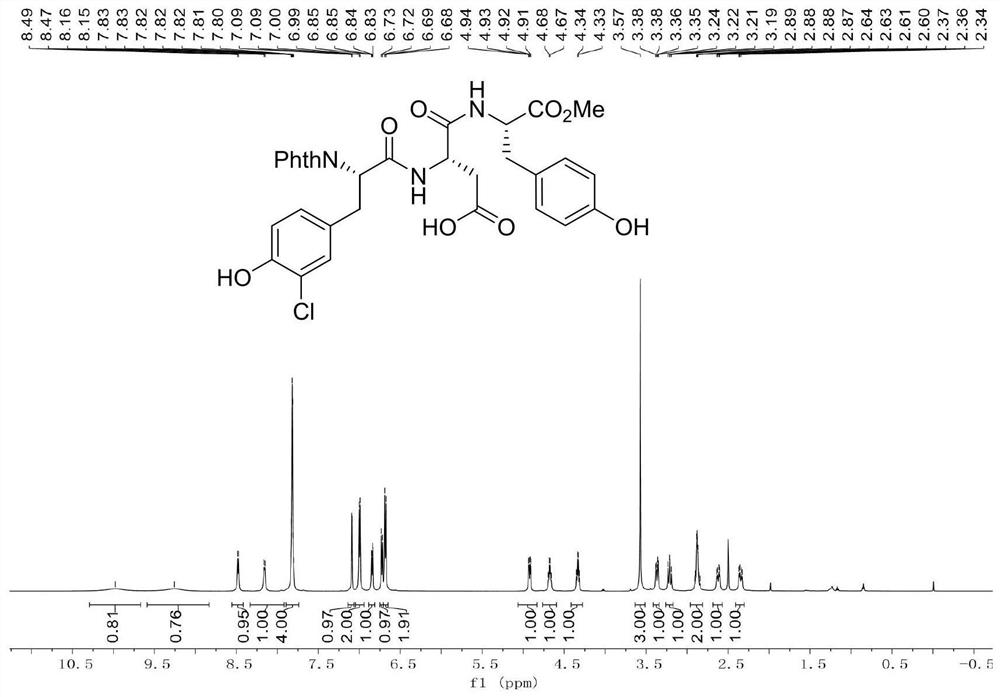
[0039] Example 1: Add dipeptide Phth-L-Ala-Asp(OH)-OMe (696.6mg, 2.0mmol), 4-iodophenol (880.2mg, 4.0mmol), catalyst 4 into a clean and dry 10mL Shrek tube (Triphenylphosphine) palladium (231.1mg, 10mol%), oxidant silver trifluoroacetate (342.1mg, 3.0mmol), additive potassium dihydrogen phosphate (544.4mg, 4.0mmol) and toluene (4.0mL), 20 ℃ air React in the atmosphere for 12 hours. After the reaction was completed, the reaction was cooled to room temperature, then diluted with 10 mL of ethyl acetate, concentrated under reduced pressure to remove the solvent, and purified by silica gel column chromatography (the elution solvent was CH 2 Cl 2 :MeOH:AcOH=100:2:1, volume ratio) to obtain 768.1 mg of a white solid product (ie product 1), with a yield of 87.2%.
[0040] Data characterization of dipeptide raw material Phth-L-Ala-Asp(OH)-OMe:
[0041]
[0042] M.p.=133-135℃.
[0043] 1 H NMR (400MHz, CDCl 3 )δ9.80(br,1H),7.87-7.83(m,2H),7.75-7.71(m,2H),7.07(d,J=8.0Hz,1H),4.95...
Embodiment 2
[0054] Example 2: The experimental process of Example 2 was repeated in Example 1, except that the "reaction time was adjusted to 24 hours", and finally 791.0 mg of white solid product was obtained, with a yield of 89.8%.
Embodiment 3
[0055] Example 3: The experimental process of Example 3 was repeated in Example 1, the only difference being that "reaction time was adjusted to 48 hours", and finally 791.9 mg of white solid product was obtained, with a yield of 89.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com