Protein kinase degradation agent and application thereof
A technology of selecting compounds, applied in medical preparations containing active ingredients, metabolic diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
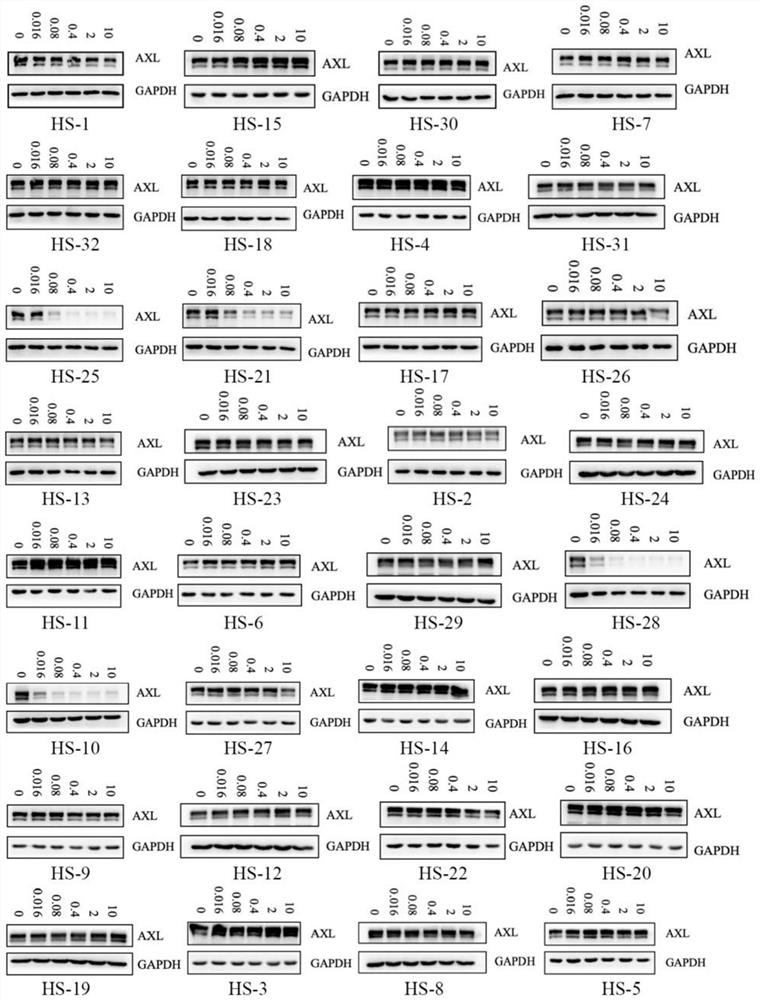
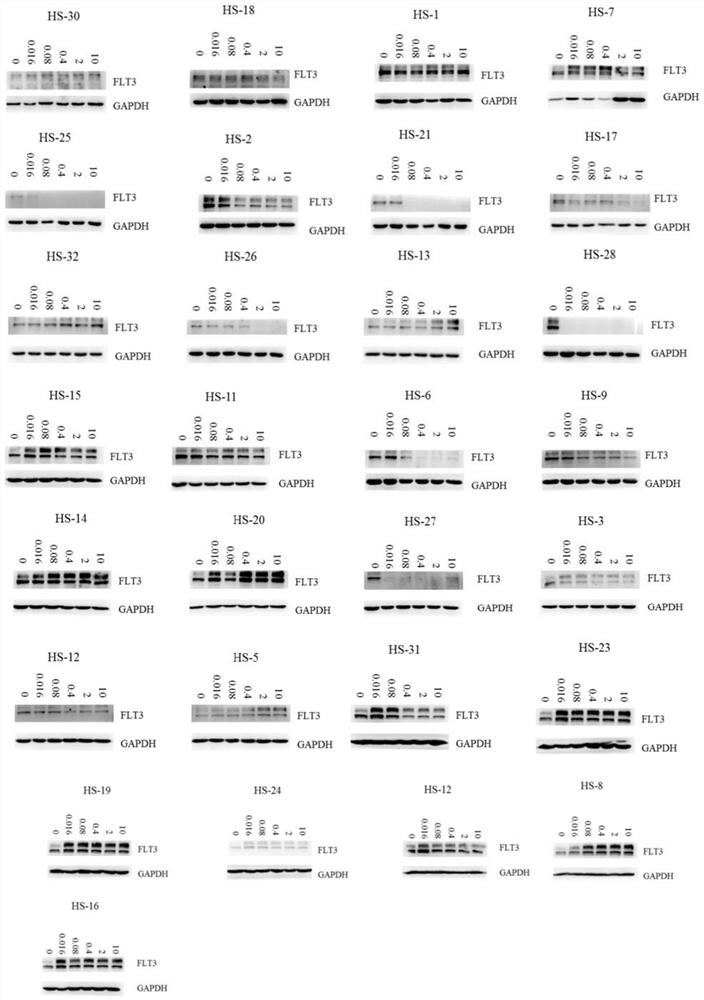
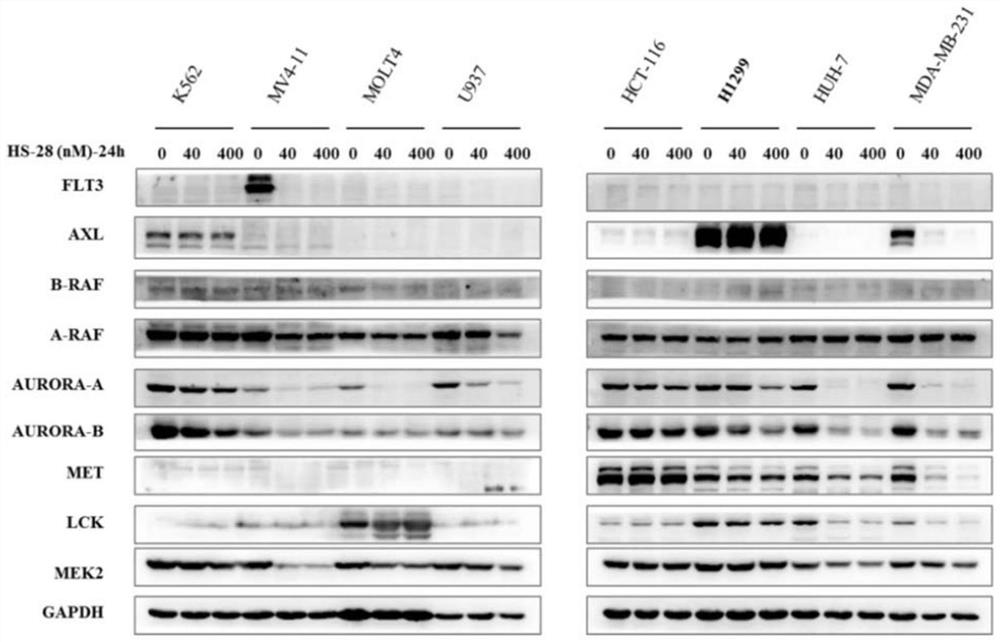
Image
Examples
Embodiment 1
[0150] Example 1: N-(4-((7-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindoline-4-yl )amino)-2-oxyethyl)-6-methylquinazolin-4-yl)oxy)-3-fluorophenyl)-6-ethyl-1,2-dimethyl-4-oxo Substituent-1,4-dihydroquinoline-3-carboxamide (HS-1)
[0151]
[0152]
[0153] Step 1: Preparation of diethyl 2-(1-(((4-ethylphenyl)amino)ethylene)malonate (2)
[0154]
[0155] p-Ethylaniline (2.42g, 20mmol) and dimethyl acetylmalonate (2.02g, 10mmol) were dissolved in 50mL of n-pentane, a catalytic amount of p-toluenesulfonic acid (20mg) was added, and the mixture was refluxed overnight. Cool down to room temperature, add a small amount of saturated NaHCO 3 , extracted twice with EA, combined the organic phases, washed once with saturated brine, anhydrous Na 2 SO 4 It was dried, filtered and spin-dried, and 2.68 g (87.8%) of solid was obtained by column chromatography. 1 H NMR(400MHz,d6-DMSO)δ10.98(s,1H),7.23(d,J=8.0Hz,2H),7.14(d,J=8.0Hz,2H),4.10(m,4H),2.63 -2.58 (q, J=8.0Hz, 2H), 2....
Embodiment 2
[0177] Example 2: N-(4-((7-((6-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-yl) Amino)-6-oxohexyl)oxy)-6-methoxyquinazolin-4-yl)oxy)-3-fluorophenyl)-6-ethyl-1,2-dimethyl-4 -Oxo-1,4-dihydroquinoline-3-carboxamide (HS-2)
[0178]
[0179] The synthesis method is as in Example 1.
[0180] 1H NMR (400MHz, DMSO-d6) δ11.02(d, J=7.3Hz, 2H), 9.80(s, 1H), 8.56(s, 1H), 8.09(d, J=2.2Hz, 1H), 7.96 (dd,J=12.9,2.3Hz,1H),7.86-7.78(m,2H),7.67(dd,J=8.9,2.3Hz,1H),7.59(s,1H),7.51(d,J=1.7 Hz, 3H), 7.48-7.40(m, 2H), 5.15(dd, J=13.3, 5.1Hz, 1H), 4.46-4.31(m, 2H), 4.24(t, J=6.5Hz, 2H), 3.98 (s,3H),3.84(s,3H),2.91(ddd,J=17.7,13.4,5.4Hz,1H),2.78(q,J=7.6Hz,2H),2.65(s,3H),2.60( d,J=17.5Hz,1H),2.43(t,J=7.4Hz,2H),2.35(dd,J=13.1,4.5Hz,1H),2.02(dd,J=12.0,6.5Hz,1H), 1.88(t,J=7.4Hz,2H),1.79-1.67(m,2H),1.60-1.49(m,2H),1.25(t,J=7.5Hz,3H). 13 C NMR (151MHz, DMSO) δ173.93, 173.28, 171.77, 171.51, 168.30, 166.19, 164.55, 155.75, 154.79, 153.17, 152.55, 152.23, 150.84, 149.43, 139.98, 138.689, 139.689...
Embodiment 3
[0181] Example 3: N-(4-((7-((6-((2-(2,6-dioxopiperidin-3-yl)-3-oxoisoindoline-4-yl) Amino)-6-oxohexyl)oxy)-6-methoxyquinazolin-4-yl)oxy)-3-fluorophenyl)-6-ethyl-1,2-dimethyl-4 -Oxo-1,4-dihydroquinoline-3-carboxamide (HS-3)
[0182]
[0183] The synthesis method is as in Example 1.
[0184] 1 H NMR(400MHz,DMSO-d6)δ11.06(s,1H),10.99(s,1H),10.31(s,1H),8.55(s,1H),8.33(d,J=8.3Hz,1H) ,8.09(d,J=2.2Hz,1H),7.97(dd,J=13.0,2.3Hz,1H),7.83(d,J=8.9Hz,1H),7.68(dd,J=8.9,2.3Hz, 1H),7.62-7.54(m,2H),7.53-7.39(m,3H),7.31-7.23(m,1H),5.09(dd,J=13.2,5.2Hz,1H),4.47(d,J= 17.7Hz, 1H), 4.36(d, J=17.7Hz, 1H), 4.22(t, J=6.5Hz, 2H), 3.98(s, 3H), 3.84(s, 3H), 2.89(ddd, J= 17.2,13.6,5.5Hz,1H),2.77(q,J=7.6Hz,2H),2.64(s,4H),2.49-2.34(m,3H),2.06-1.99(m,1H),1.88(t , J=7.4Hz, 2H), 1.76(t, J=7.6Hz, 2H), 1.56(d, J=7.5Hz, 2H), 1.25(s, 3H). 13 CNMR (151MHz, DMSO) δ 173.92, 173.24, 171.72, 171.22, 169.68, 166.19, 164.53, 155.76, 154.79, 153.18, 152.52, 152.24, 150.84, 149.42, 143.08, 138.98, 98, 9) ( J=28....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com