Ester-substituted heterocyclic aromatic conjugated skeleton and polymer material and application thereof
A technology of conjugated polymers and aromatic heterocycles, applied in photovoltaic power generation, organic chemistry, etc., can solve the problems of inability to perform photoacoustic imaging and low LUMO energy level, and achieve excellent light absorption performance in the long-wave range, excellent imaging effect, The effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
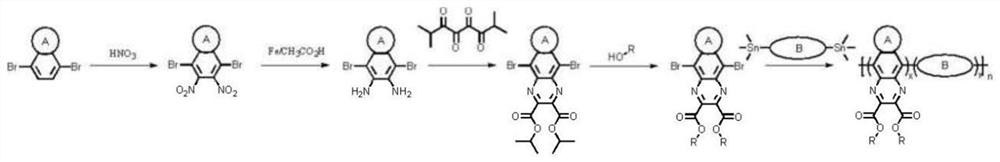
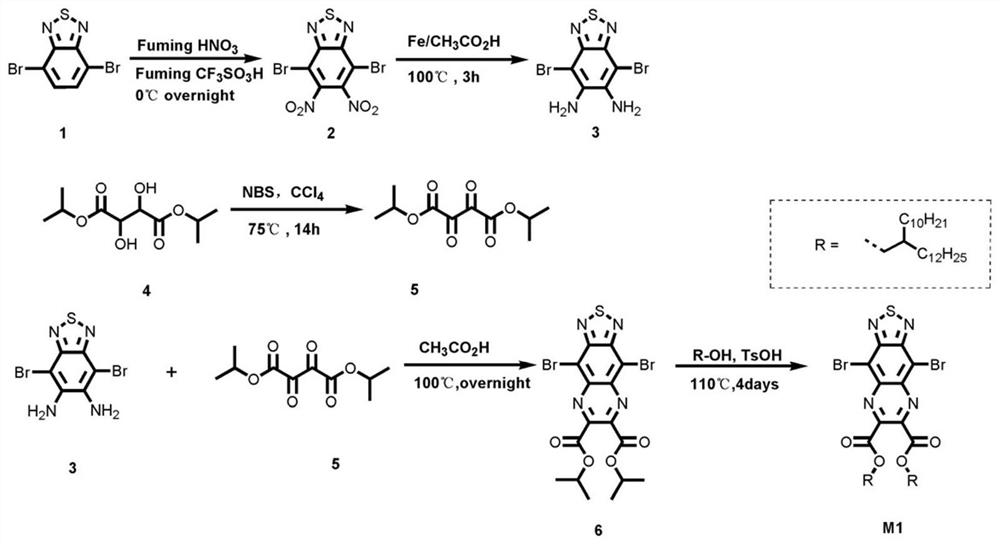
[0052] Synthesis of a conjugated skeleton of aromatic heterocyclic ring containing ester group substitution:
[0053] Its synthetic route is as follows figure 2 Shown, the reaction steps are as follows:
[0054] 1) Synthesis of product (2) 4,7-dibromo-5,6-dinitrobenzothiadiazole: Add (15 mL) fuming trifluoromethanesulfonic acid to a round-bottomed flask equipped with a stirring bar, Keeping the temperature at 0°C, 4,7-dibromobenzothiadiazole (1.000 g, 3.40 mmol) was added in small amounts and finally fuming nitric acid (15 mL) was added dropwise. The reaction was warmed to room temperature and stirred overnight;
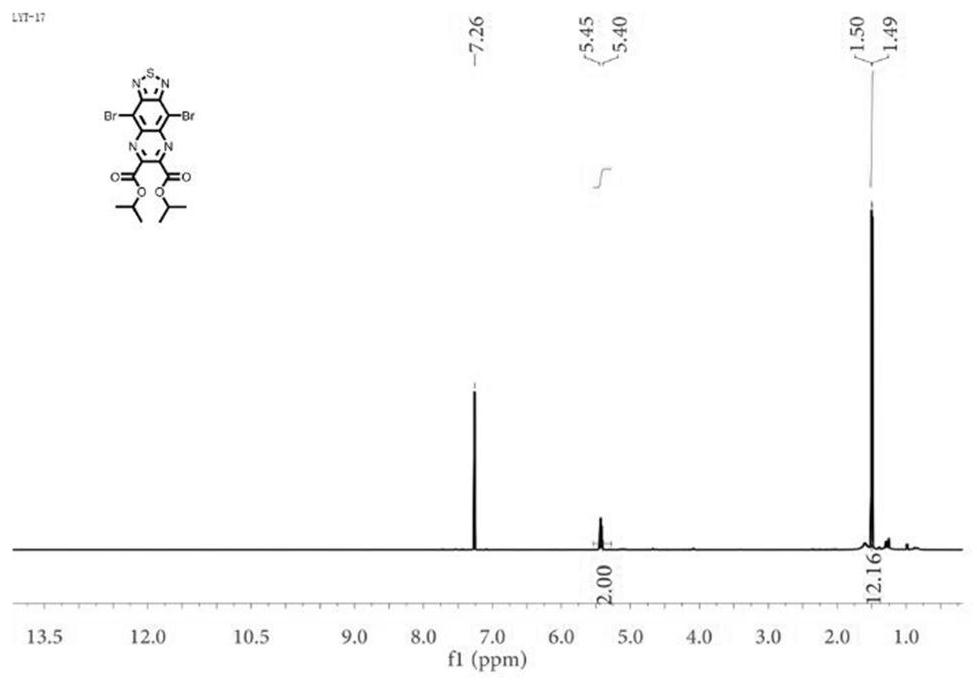
[0055] Analysis by thin layer chromatography showed disappearance of the starting material, and the reaction mixture was slowly poured into a beaker with ice / water, and the solid precipitate was recovered by filtration, washed several times with water and dried under reduced pressure. The crude product was purified by silica gel column chromatography, and a light...
Embodiment 2
[0061] A kind of synthesis of aromatic heterocyclic conjugated polymer (PATQ-T) containing ester group substitution:
[0062] Its synthetic route is as follows Figure 7 As shown, under the protection of nitrogen, the product (M1) (0.1mmol, 110.7mg) prepared in Example 1 and 2,5-bis(trimethyltin)thiophene (0.1mmol, 41mg), tri(dimethoxy Benzylacetone) Dipalladium (Pd 2 (dba) 3 ) (2.75mg, 0.003mmol) and three (o-methylphenyl) phosphorus (P (o-Tol) 3 ) (3.65mg, 0.012mmol) was dissolved in 5mL of chlorobenzene solution. Under the protection of nitrogen, the reaction was refluxed for 24 hours. After cooling to room temperature, the reaction liquid was dropped into 150 mL of methanol solution to precipitate, and filtered to obtain a green solid. After extracting with acetone for 24 hours in a Soxhlet extractor, the final polymer was washed with tetrahydrofuran, cooled and filtered through a 0.45 μm organic membrane, precipitated in methanol, filtered, and vacuum-dried. The dark...
Embodiment 3
[0064] A kind of synthesis of aromatic heterocyclic conjugated polymer (PATQ-Se) containing ester group substitution:
[0065] Its synthetic route is as follows Figure 8 As shown, the product (M1) and 2,5-bis(trimethyltin)selenophene obtained in Example 1 are selected as polymerized monomers, and all the other specific operations are the same as in Example 2 to obtain dark green solid polymer PATQ-Se , yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com