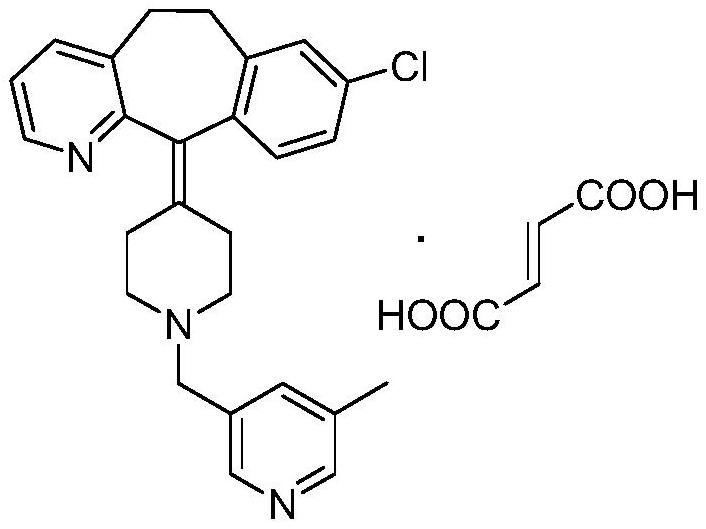
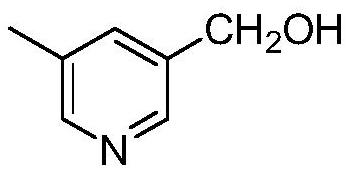
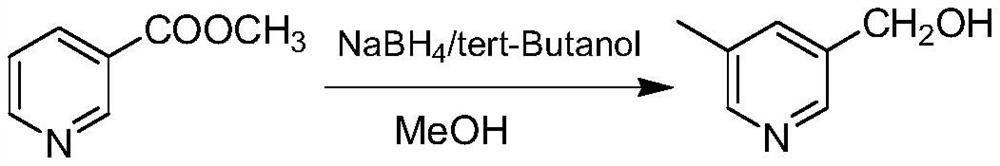Preparation method of rupatadine fumarate intermediate 5-methyl-3-hydroxymethylpyridine
A technology of rupatadine fumarate and hydroxymethyl pyridine, which is applied in the field of preparation of pharmaceutical compound intermediates, can solve the problems of complicated operation, flushing of reaction liquid, endangering the life safety of operators and the like, and achieves high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] First add 70mL of tert-butanol into the reaction flask, then add 20g of sodium borohydride, heat to 51°C and stir to mix evenly. Then add the methanol solution of methyl 5-methylnicotinate (25g of methyl 5-methylnicotinate dissolved in 60mL of methanol solution) dropwise into the mixture of tert-butanol and sodium borohydride, and heat up to 70°C to react and reflux 3h. After the reaction, the reaction solution was cooled to room temperature, and 50 mL of purified water was added successively, stirred for 15 minutes, and 160 mL of 6N hydrochloric acid solution, stirred thoroughly. Concentrate under reduced pressure to remove 60%-70% solvent, add 10mol / L sodium hydroxide solution until the pH is about 11, and cool to room temperature. The organic phase was separated by extraction with 100 mL of dichloromethane, concentrated under reduced pressure to dryness of dichloromethane to obtain 18.5 g of yellow oil with a yield of 91%. 99.3% purity.
Embodiment 2
[0038] First add 160mL of tert-butanol into the reaction flask, then add 29.8g of sodium borohydride, heat to 51°C and stir to mix evenly. Then add the methanol solution of 5-methylnicotinate methyl ester (36g of 5-methylnicotinic acid methyl ester dissolved in 80mL methanol solution) dropwise to the above-mentioned mixture of tert-butanol and sodium borohydride, and heat up to 90°C to react and reflux 3h. After the reaction, the reaction liquid was cooled to room temperature, and 70 mL of purified water was added successively, stirred for 15 minutes, and 230 mL of 6N hydrochloric acid solution, stirred thoroughly. Concentrate under reduced pressure to remove 60%-70% solvent, add 10mol / L sodium hydroxide solution until the pH is about 11, and cool to room temperature. The organic phase was separated by extraction with 140 mL of dichloromethane, concentrated under reduced pressure to dry dichloromethane to obtain 25.5 g of yellow oil, with a yield of 86.3%. 98.3% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com