Dimethyl deuterated carnosol, preparation method thereof and application of dimethyl deuterated carnosol in preparation of medicine for treating cachexia
A technology of bismethyl deuterated carnosol and bismethyl deuterated sage, applied in the field of drug synthesis, can solve problems such as failure, no breakthrough progress of new drugs, shortened survival period, etc., so as to alleviate muscle atrophy , the effect of alleviating weight and food intake loss, alleviating lipolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Example 1 Synthesis of Dimethylcarnosol Add 300mg carnosol, 250mg potassium carbonate and 20mL anhydrous acetone into a 50mL reaction flask, add 1mL methyl iodide under nitrogen protection, and react in the dark for 48 hours at room temperature. The liquid turned from colorless to brown, and the solid was removed by suction filtration. The filtrate was concentrated to dryness and separated by column chromatography to obtain 70 mg of white solid, with a yield of 21.5%. LC-MS (ESI) m / z C 22 h 31 o 4 [M+H] + calcd for 359.2, found 359.4.
Embodiment 2
[0164] The synthesis of embodiment 2 bismethyl deuterated carnosol
[0165] Add 750mg of carnosol, 600mg of potassium carbonate and 50mL of anhydrous acetone to a 100mL reaction bottle, add 3mL of deuterated methyl iodide under nitrogen protection, and react in the dark for 48 hours at room temperature. The reaction solution turns from colorless to brown, and is filtered by suction The solid was removed, the filtrate was concentrated to dryness and separated by column chromatography to obtain 135 mg of white solid with a yield of 16.3%. LC-MS (ESI) m / z C 22 h 25 D. 6 o 4 [M+H]+ calcd for 364.3, found 365.5.
Embodiment 3
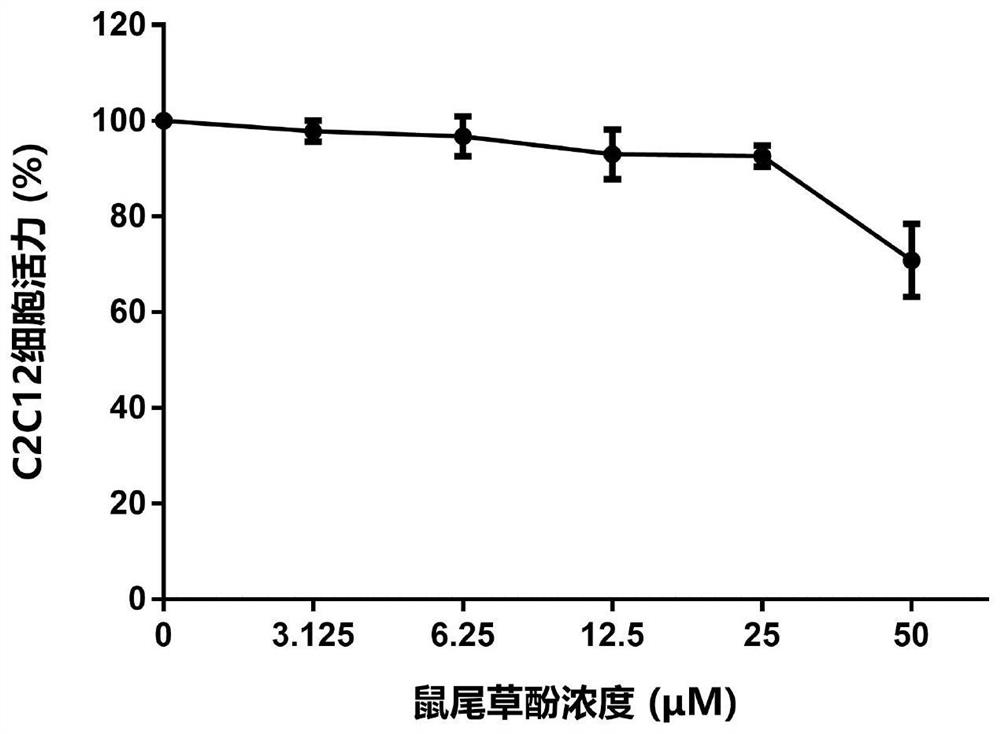
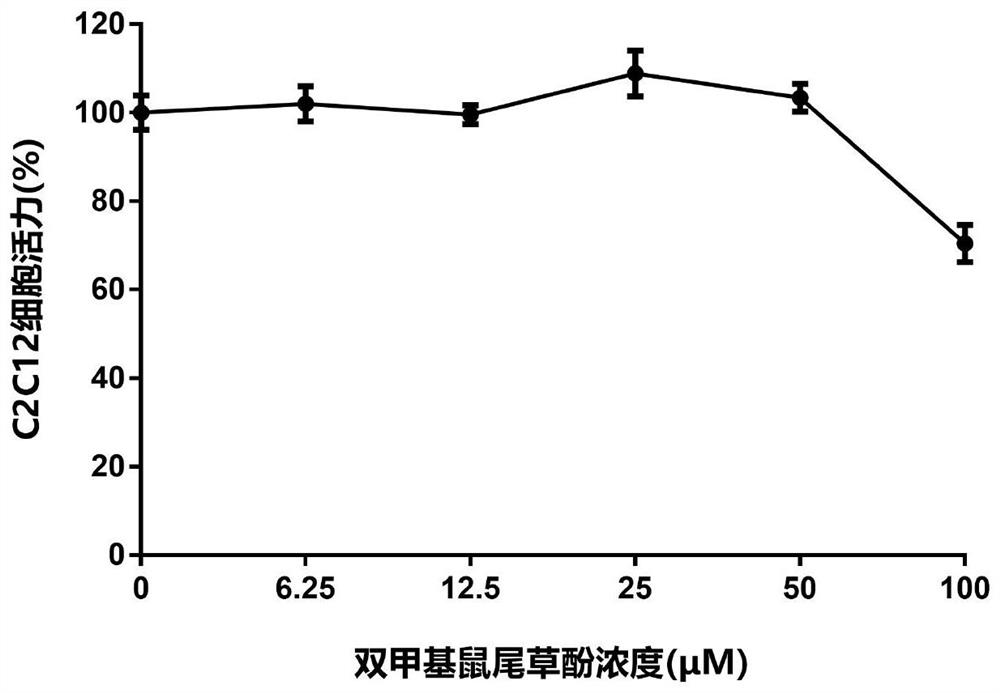
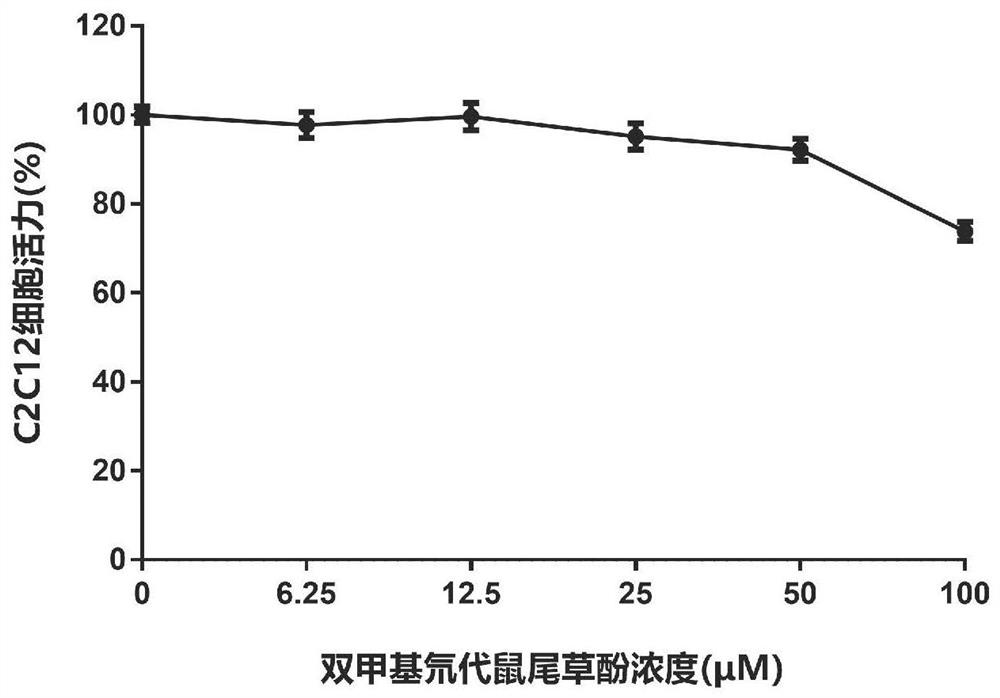
[0166] Example 3 Carnosol, dimethyl carnosol and dimethyl deuterated carnosol on mouse myoblast (C2C12) viability experiment
[0167] The survival rate of C2C12 cells was detected by MTT assay. The detection principle is that succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystal formazan (Formazan) and deposit in the cells, while dead cells do not have this function. Triple solution (10% SDS + 5% isobutanol + 0.01% concentrated hydrochloric acid, aqueous solution) can dissolve formazan in cells, and measure its light absorption value at a wavelength of 570nm with an enzyme-linked immunosorbent assay, which can indirectly reflect the number of living cells . Within a certain cell number range, the amount of MTT crystal formation is proportional to the cell number.
[0168] The effect of the drug on the viability of mouse myoblasts (C2C12) can be evaluated by the above method. The specific method is as foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com