Application of cordyceps militaris pharmaceutical composition in preparation of preparation for preventing and/or treating novel coronaviruses and resisting respiratory viruses
A kind of anti-respiratory, Cordyceps militaris technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In view of the problem that there is no effective treatment drug for respiratory virus infection, this embodiment provides a Cordyceps militaris medicinal composition for prevention and treatment of respiratory virus.
[0038] Because the common pathogenic coronaviruses and influenza viruses of the respiratory tract are RNA viruses, the RNA Coxsackie B3 virus (CVB3, Nancy strain, Shanghai Public Health Clinical Center) with no biosafety risk is used as a research model. It has been confirmed horizontally that the Cordyceps militaris extract containing cordycepin and pentostatin can effectively inhibit the replication of CVB3 virus in its susceptible human malignant embryonic rhabdomyoma cells (RD), and protect the cells from the pathogenic effect of the virus. Low concentrations of cordycepin combined with pentostatin also have the same antiviral effect.
[0039] Further, the pharmaceutical composition of Cordyceps militaris provided in this embodiment includes the extr...
Embodiment 2
[0042] This example is an antiviral activity experiment of high concentration cordycepin alone.
[0043] The experimental method includes the following steps:
[0044] Step 1, adjust the concentration of human malignant embryonic rhabdomyoma cells (RD) to 3×10 with DMEM cell culture fluid containing 10% fetal bovine serum (FBS). 5 / mL, uniformly inoculated at 1 mL per well in a 12-well plate and cultured overnight.
[0045] Step 2, after diluting cordycepin with DMEM culture medium, different final concentrations of cordycepin-containing solutions (1ug / mL, 10ug / mL, 20ug / mL, 30ug / mL) were obtained. The experiment was divided into blank control group and experimental group. Treat the RD cells with the blank control group (only containing DMEM culture medium) and the four experimental groups (containing the above four solutions containing cordycepin), and infect the Coxsackie virus type B3 at a ratio of 2 (MOI=2) (CVB3) RNA virus. After 1 hour of virus infection, the virus li...
Embodiment 3
[0049] This example provides an antiviral experiment of low concentration cordycepin combined with pentostatin.
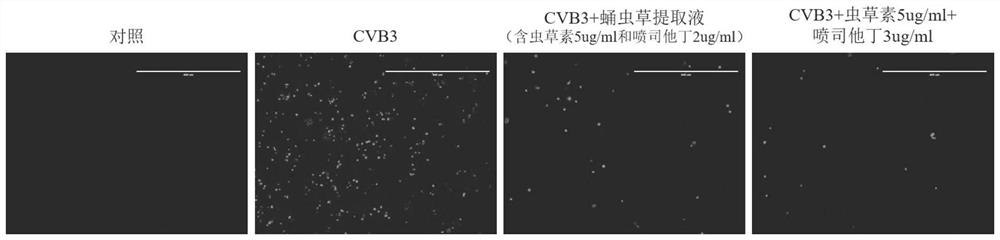
[0050] Experimental method: The experimental method provided in this example is similar to that in Example 2, the only difference is that the experimental group is divided into eight groups, namely: the final concentration of 0.1ug / mL cordycepin alone group, the final concentration of 1ug / mL cordycepin Single use group, final concentration of 5ug / mL cordycepin single use group, final concentration of 30ug / mL cordycepin single use group, final concentration of 3ug / mL pentostatin single use group, final concentration of 0.1ug / mL Cordycepin + 3ug / mL pentostatin combined group, the final concentration is 1ug / mL cordycepin + 3ug / mL pentostatin combined group, the final concentration is 5ug / mL cordycepin + 3ug / mL pentostatin Joint use group.
[0051] Experimental results such as figure 2 shown.
[0052] figure 2 It is the antiviral experiment result of the combinat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com