Stem cell serum-free culture medium and preparation method and application thereof
A serum-free medium and stem cell technology, applied in the field of stem cell serum-free medium and its preparation, can solve the problem that mesenchymal stem cell-related products cannot meet clinical treatment requirements, high risk of allogeneic immune rejection, and unstable cultured cell quality, etc. It can protect and maintain growth and proliferation in vitro, improve the speed of in vitro culture, and avoid unfavorable factors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] In a second aspect, the present invention provides a method for preparing a serum-free medium for stem cells, comprising the following steps: adding platelet lysate, transferrin solution and human insulin solution to the basal medium to prepare the serum-free medium for stem cells.
[0051] The preparation method of the stem cell serum-free medium provided by the invention is simple and convenient.
[0052] As a further technical solution, before adding the transferrin solution and the human insulin solution to the basal medium, it also includes the step of filtering the transferrin solution and the human insulin solution to remove the impurities in the transferrin solution and the human insulin solution. of insoluble substances to obtain a homogeneously dissolved solution.
[0053] The filtering includes filtering with a filter membrane of 0.20 μm to 0.25 μm;
[0054] Preferably, a 0.22 μm filter membrane is used for filtration.
[0055] In the third aspect, the pres...
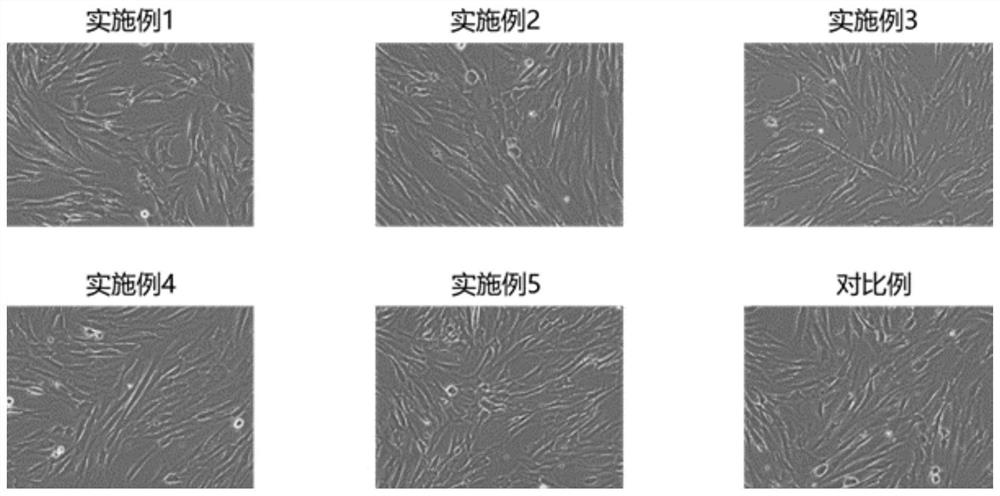
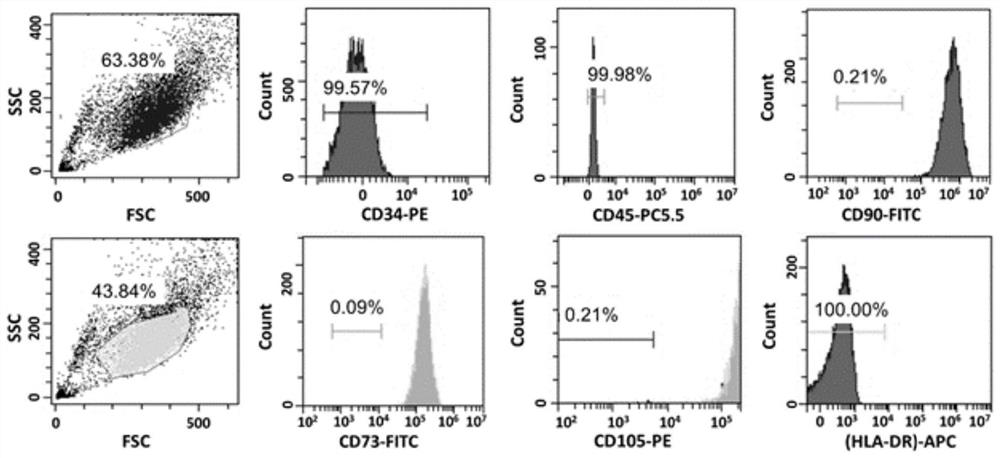
Embodiment 1
[0064] A stem cell serum-free medium, consisting of α-MEM medium and additional components, the additional components are platelet lysate, recombinant transferrin and recombinant human insulin, wherein the volume of platelet lysate in the stem cell serum-free medium The percentage is 5%, the concentration of recombinant transferrin is 5 μg / mL, and the concentration of recombinant human insulin is 5 μg / mL.
[0065] The preparation method is as follows:
[0066] Dissolve recombinant human insulin with 10mM hydrochloric acid to a concentration of 1mg / mL, pass through a 0.22 micron filter membrane, and add it to the basal medium at a final concentration of 5μg / mL; dissolve recombinant transferrin with sterile water to a concentration of 1mg / mL, after passing through a 0.22 micron filter membrane, it was added to the basal medium at a final concentration of 5 μg / mL; the platelet lysate was added to the above medium, and the volume ratio of the platelet lysate to the above medium w...
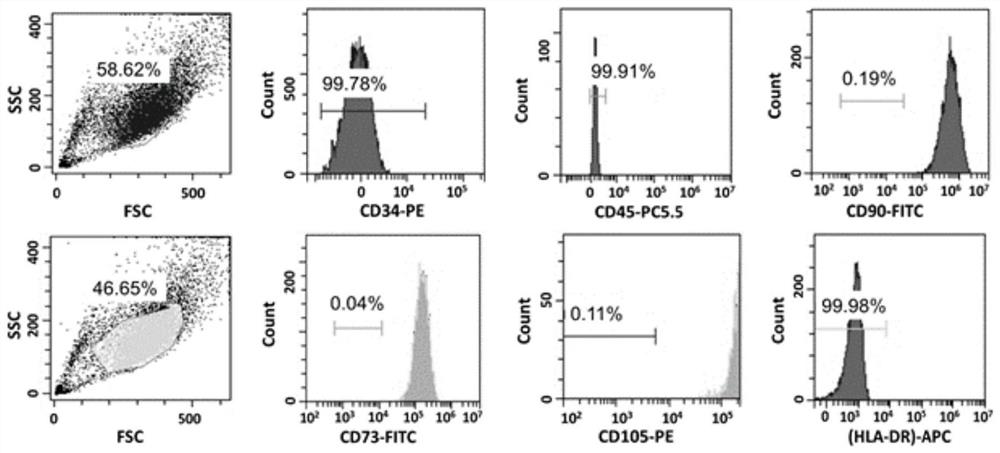
Embodiment 2
[0068] A stem cell serum-free medium, consisting of α-MEM medium and additional components, the additional components are platelet lysate, recombinant transferrin and recombinant human insulin, wherein the volume of platelet lysate in the stem cell serum-free medium The percentage is 5%, the concentration of recombinant transferrin is 10 μg / mL, and the concentration of recombinant human insulin is 10 μg / mL.
[0069] The preparation method is as follows:
[0070] Dissolve recombinant human insulin with 10mM hydrochloric acid to a concentration of 1mg / mL, pass through a 0.22 micron filter membrane, and add it to the basal medium at a final concentration of 10μg / mL; dissolve recombinant transferrin with sterile water to a concentration of 1mg / mL, after passing through a 0.22 micron filter membrane, it was added to the basal medium at a final concentration of 10 μg / mL; the platelet lysate was added to the above medium, and the volume ratio of the platelet lysate to the above medi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com