Alkynylated tetrahydroisoquinoline compound as well as preparation method and application thereof
A tetrahydroisoquinoline and compound technology, which is applied in the directions of organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problems of poor compatibility of alkyl alkynes, dangerous storage, complicated reaction operation, etc., and achieves novel structure and good biological activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1 of the present invention is: a preparation method of alkynylated tetrahydroisoquinoline compounds, comprising the following steps:
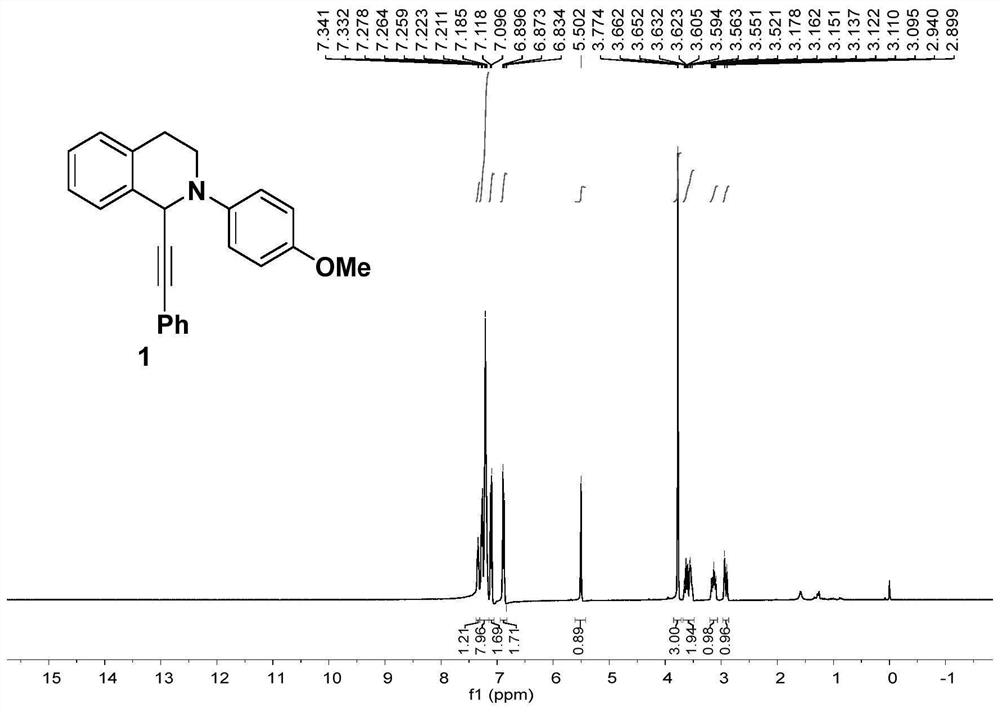
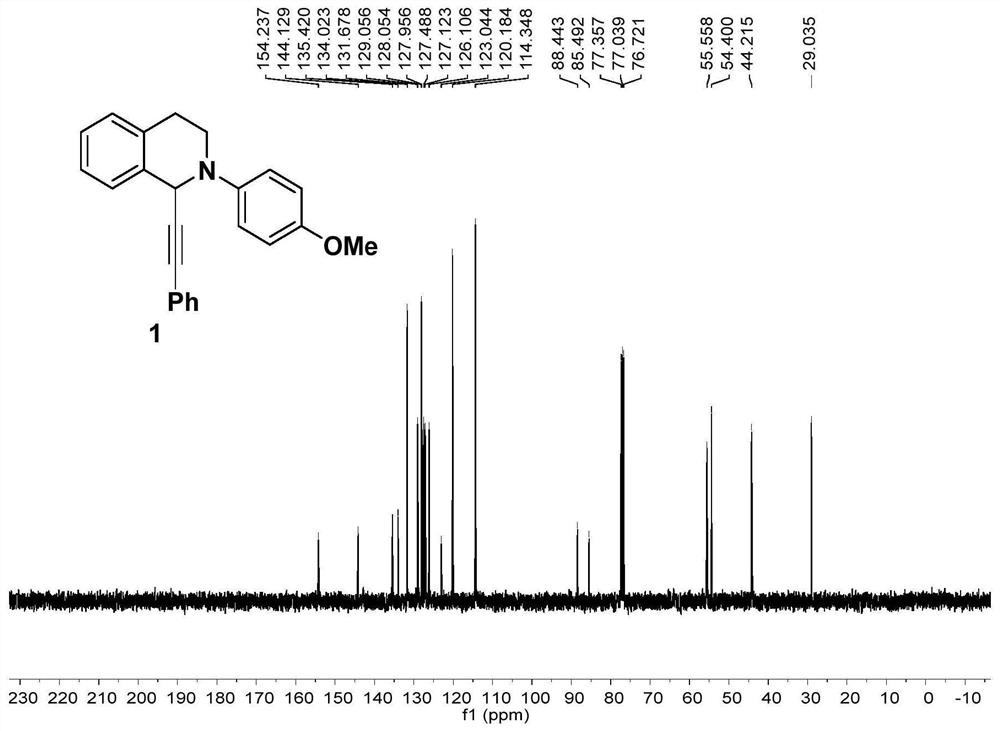
[0077] In an undivided electrolyzer, add Cu(OTf) 2 (10mol%) and ligand L1 4,4'-tert-butylbipyridine (10mol%), n-Bu 4 NPF 6 (0.2mmol), tetrahydroisoquinoline 1a (0.2mmol), phenylpropylic acid 1b (0.24mmol), Et 3 N (0.24mmol), anhydrous MeCN (4mL), then insert platinum and nickel electrodes respectively as the anode and cathode of the reaction, electrolyze at a constant current of 1.5mA for 10h, after the reaction is completed, extract with ethyl acetate and collect the organic phase , and then the organic phases were mixed, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product, which was purified by silica gel column chromatography to obtain compound 1 with a yield of 85%.
[0078] The characterization results of Compound 1 prepared in Example 1 of the present invention are ...
Embodiment 2
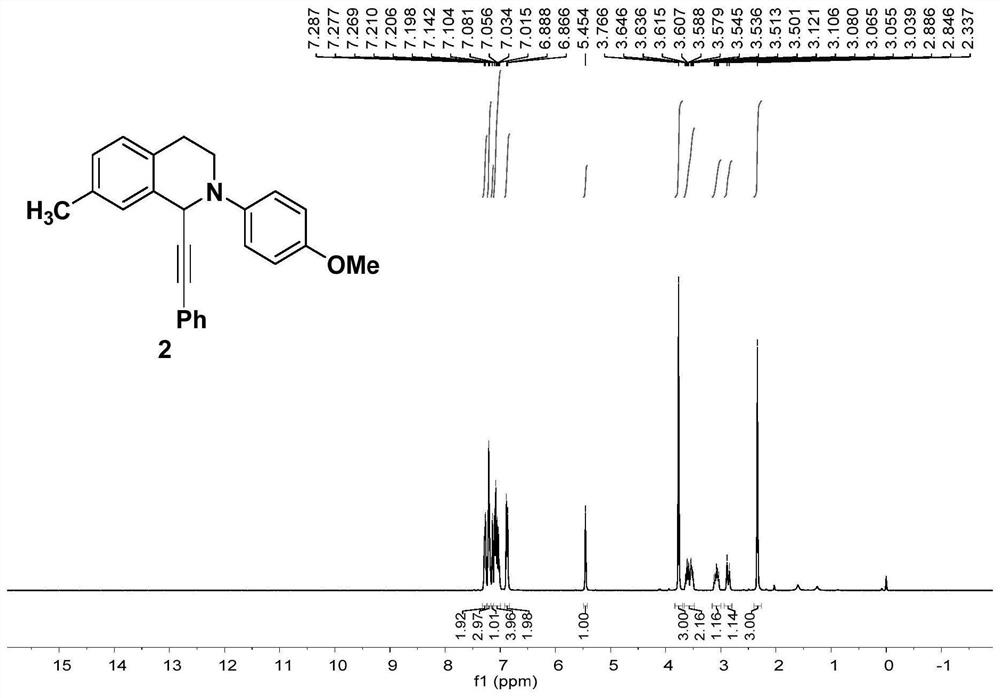
[0081] Embodiment 2 of the present invention is: a preparation method of alkynylated tetrahydroisoquinoline compounds, the difference from Embodiment 1 is that the ligand is replaced by L 2 4,4'-methoxybipyridine, the yield of compound 1 was 80%.
[0082]
Embodiment 3
[0083] Embodiment 3 of the present invention is: a preparation method of alkynylated tetrahydroisoquinoline compounds, the difference from Embodiment 1 is that the ligand is replaced by L 3 2,2'-methylbipyridine, the yield of compound 1 was 2%.
[0084]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com