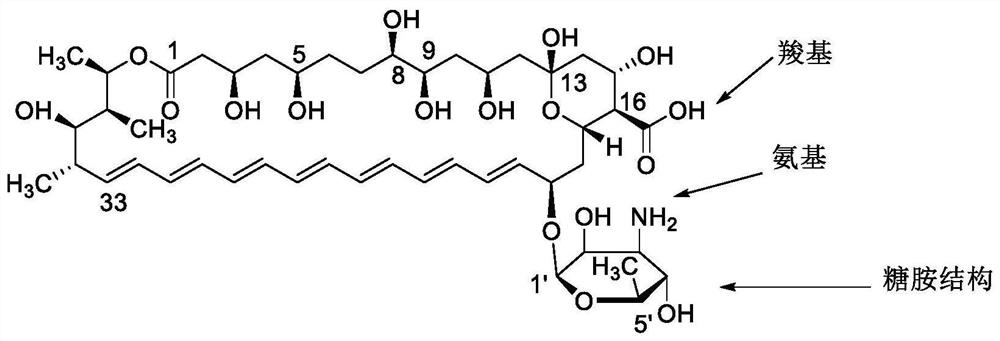
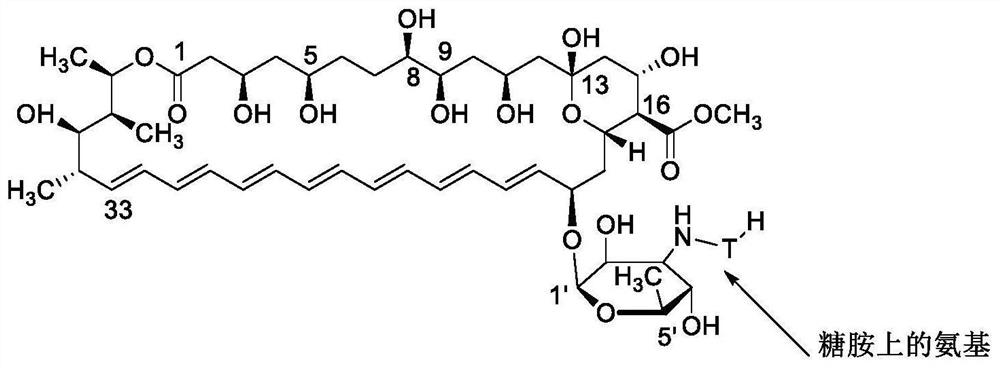
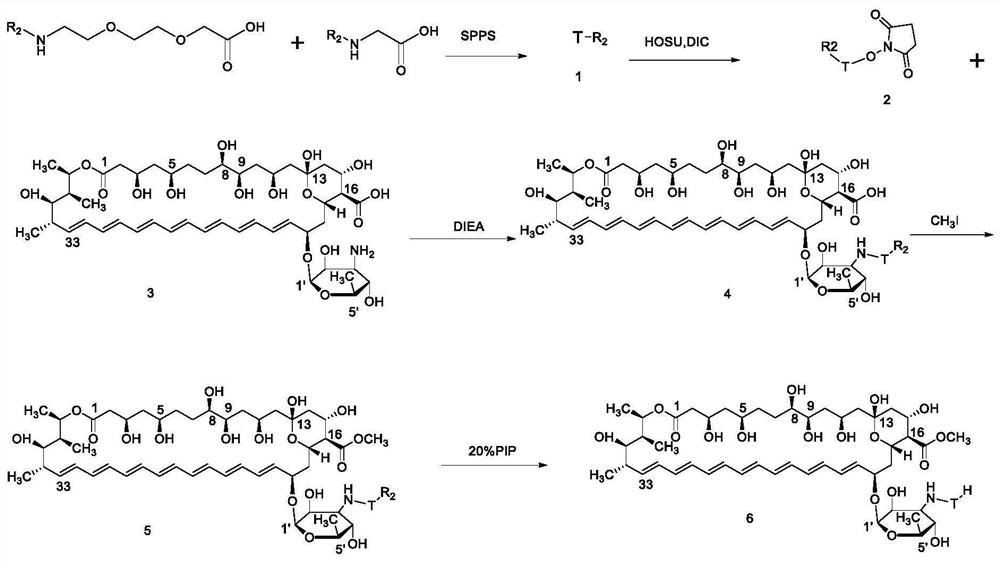
Amphotericin B methyl ester peptide derivative and preparation method thereof
A technology of derivatives and methyl groups, applied in the field of amphotericin B methyl esterified peptide derivatives and their synthesis and preparation, can solve the problems of liposome instability, increased therapeutic dose, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1: Preparation and purification of DMR005-JZH
[0182] Hydrophilic peptide structure: Fmoc-AEEAc-AEEAc-AEEAc-AEEAc–AEEAc-OH
[0183] DMR005-JZH structure:
[0184]
[0185] (1) Materials and reagents
[0186] 2-CTC resin, substitution value 0.945mmol / g.
[0187] The amino acid is: Fmoc-AEEAc-OH
[0188] Synthetic reagents: HATU, DMF, DCM, DIEA, piperidine, methyl iodide.
[0189] (2) Instrument
[0190] CS-BIO peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, Beckman centrifuge, Buchi rotary evaporator.
[0191] (3) Operation steps (take 1g resin as an example)
[0192] a. Solid-phase chemical synthesis of peptides
[0193] Weigh 1.00g of 2-CTC resin, put it in the reactor of polypeptide synthesizer, add 10mL DCM, soak for 1h, weigh 2-3 times the amount of Fmoc-AEEAc-OH and absorb 4-6 times the amount of DIEA and add 10mL DCM to dissolve it , put it into the reactor, and carry out the reaction. The reaction temper...
Embodiment 2
[0218] Example 2: Preparation and purification of DMR078-JZH
[0219] Hydrophilic peptide structure: Fmoc-AEEAc-Gly-AEEAc-Gly–AEEAc-OH
[0220] DMR078-JZH structure:
[0221]
[0222] (1) Materials and reagents
[0223] 2-CTC resin, substitution value 0.945mmol / g.
[0224] The amino acids are: Fmoc-AEEAc-OH and Fmoc-Gly-OH
[0225] Synthetic reagents: HATU, DMF, DCM, DIE, piperidine, methyl iodide.
[0226] (2) Instrument
[0227] CS-BIO peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, Beckman centrifuge, Buchi rotary evaporator.
[0228] (3) Operation steps (take 1g resin as an example)
[0229] a. Solid-phase chemical synthesis of peptides
[0230] Weigh 1.00g of 2-CTC resin, put it in the reactor of polypeptide synthesizer, add 10mL DCM, soak for 1h, weigh 2-3 times the amount of Fmoc-AEEAc-OH and absorb 4-6 times the amount of DIEA and add 10mL DCM to dissolve it , put it into the reactor, and carry out the reaction. The re...
Embodiment 3
[0256] Example 3: Preparation and purification of DMR086
[0257] Hydrophilic peptide structure: Fmoc-AEEAc-AEEAc-OH
[0258] DMR086 structure:
[0259]
[0260] (1) Materials and reagents
[0261] 2-CTC resin, substitution value 0.945mmol / g.
[0262] Amino acid: Fmoc-AEEAc-OH,
[0263] Synthetic reagents: HATU, DMF, DCM, DIEA, piperidine, methyl iodide.
[0264] (2) Instrument
[0265] CS-BIO peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, Beckman centrifuge, Buchi rotary evaporator.
[0266] (3) Operation steps (take 1g resin as an example)
[0267] a. Solid-phase chemical synthesis of peptides
[0268]Weigh 1.00g of 2-CTC resin, place it in a polypeptide synthesizer reactor, add 10mL DCM, soak for 1h, weigh 2-3 times the amount of Fmoc-AEEA-OH and absorb 4-6 times the amount of DIEA and add 10mL DCM After dissolving, put it into the reactor and carry out the reaction. The reaction temperature is room temperature (25°C and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com