New use of ebselen
A technology of ebselen and its use, which is applied in the field of drugs for the treatment of type I mucopolysaccharidosis, and can solve the problems of expensive treatment and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
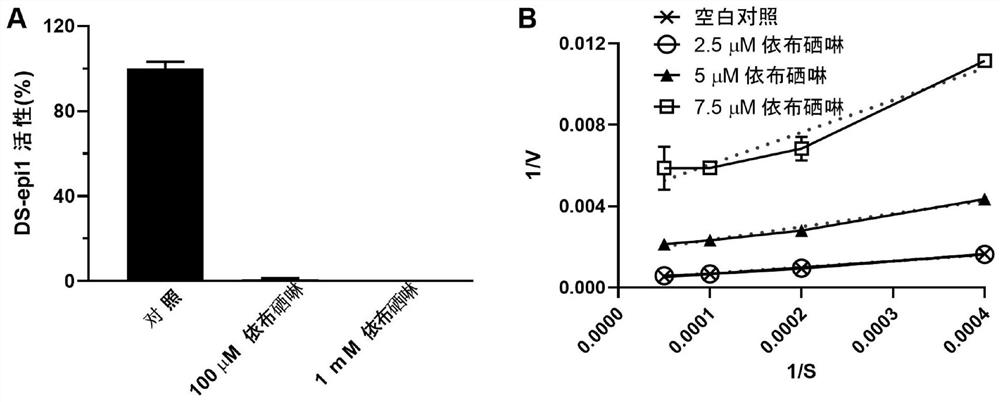
[0038] Example 1: Ebselen inhibits the activity of GAG synthesis epimerases HS-epi and DS-epi1
[0039] Enzyme activity test:
[0040] DS-epi1 activity in 100 μl buffer (20mM MES, pH 5.5, 10% glycerol, 2mMMnCl 2 ), using 30000dpm substrate [5- 3 H]dK4 (substrate prepared according to (Hannesson, H.H., A. Hagner-McWhirter, K. Tiedemann, U. Lindahl, and A. Malmstrom. 1996. Biochem J, 313 (Pt 2): 589-96.), test Method adapted from (Maccarana, M., B. Olander, J. Malmstrom, K. Tiedemann, R. Aebersold, U. Lindahl, J.P. Li, and A. Malmstrom. 2006. J Biol Chem. 281:11560-8.) ). After incubation at 37°C for 16-20 hours, 90 μl of the mixture was added to a scintillation vial (Campbell, P. ., H.H. Hannesson, D. Sandback, L. Roden, U. Lindahl, and J.P. Li. 1994. J Biol Chem. 269:26953-8). Vials were spun down for 30 seconds and equilibrated for at least 6 hours prior to scintillation counting of radioactivity. Background≤200dpm.
[0041] The activity of HS-epi was tested in 50μl co...
Embodiment 2
[0048] Example 2: Effect of ebselen on MPS-I fibroblast synthesis and catabolism of GAG in vitro
[0049] Cell culture:
[0050]Primary human dermal fibroblasts derived from a 1-year-old MPS-I patient and a healthy age-matched donor were purchased from the “Cell Lines and DNA Biobank from Patients Affected by Genetic Diseases” (Genova, Italy Gaslini Institute). MPS-I fibroblasts have the stop codon pW402X in the iduronidase gene, the most common MPS-I variant for this mutation (31% worldwide) (Kubaski, F., F.de et al . 2020. Diagnostics (Basel), 10.). Detection of fibroblast lysates (Ou, L., T.L. Herzog, C.M. Wilmot, and C.B. Whitley. 2014) with the fluorogenic substrate 4-methylumbelliferyl-α-L-idosyl (Ou et al. 2014) .Mol Genet Metab, 111:113-5.) showed that the iduronidase activity was significantly reduced (5000 times less in pW402X MPS-I fibroblasts than in control fibroblasts). Fibroblasts were cultured in DMEM, 10% fetal bovine serum (FBS), 100 units / ml penicillin a...
Embodiment 3
[0062] Example 3: Effects of Ebselen on Xenopus Embryo Development in Vivo
[0063] Xenopus embryo manipulation experiments:
[0064] For in vivo pharmacological inhibitory treatment, embryos were cultured from the neurula stage (stage14 or st. Incubate in saline (MBS) at 17°C, add 0.025% dimethyl sulfoxide (DMSO) alone as a control group, or add 12.5 μM ebselen together as a drug administration group. Embryos were prepared, cultured, and Lineage tracing and in situ hybridization by Red-Gal staining.
[0065] Antisense morpholino oligonucleotide (Dse-MO) (5′-GCT CCC CGA GTG TGA GTC CTC ATT G-3′, SEQ ID No.: 1) and standard control-MO (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3', SEQ ID No.: 2) was purchased from Gene Tools LLC. For synthesis of nlacZ mRNA, pCS2-nlacZ cDNA was linearized with NotI using the mMessage Machine kit (Ambion) and transcribed with Sp6 RNA polymerase. Morpholino (MO) animals were injected into all blastomeres at the two or four cell stage unless other...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com