Recombinant pichia pastoris genetically engineered bacterium as well as construction method and application thereof
A technology of genetically engineered bacteria and Pichia pastoris, applied in the fields of genetic engineering and fermentation engineering, can solve the problems of difficult immobilization fermentation and weak film-forming ability, and achieve the effect of shortening the fermentation period and enhancing the biological film-forming ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of an overexpression plasmid that overexpresses the HSF1 gene.
[0052] 1. Amplification of the target gene HSF1:
[0053] The gene HSF1 was amplified with primer 1 (SEQ ID NO.2) and primer 2 (SEQ ID NO.3) using the extracted P. Pastoris GS115 genome (purchased from Miaoling Biotech Co., Ltd.) as a template.
[0054] The PCR reaction system is shown in Table 1.
[0055] Table 1 Target gene HSF1 amplification PCR system
[0056]
[0057] Dispense into 25 μL per tube according to the above system.
[0058] PCR conditions: 1) pre-denaturation at 94°C, 5min; 2) denaturation at 98°C, 10s; 3) annealing at 55°C, 5s; 4) extension at 72°C, 15s, a total of 35 cycles; 5) complete extension at 72°C, 10min.
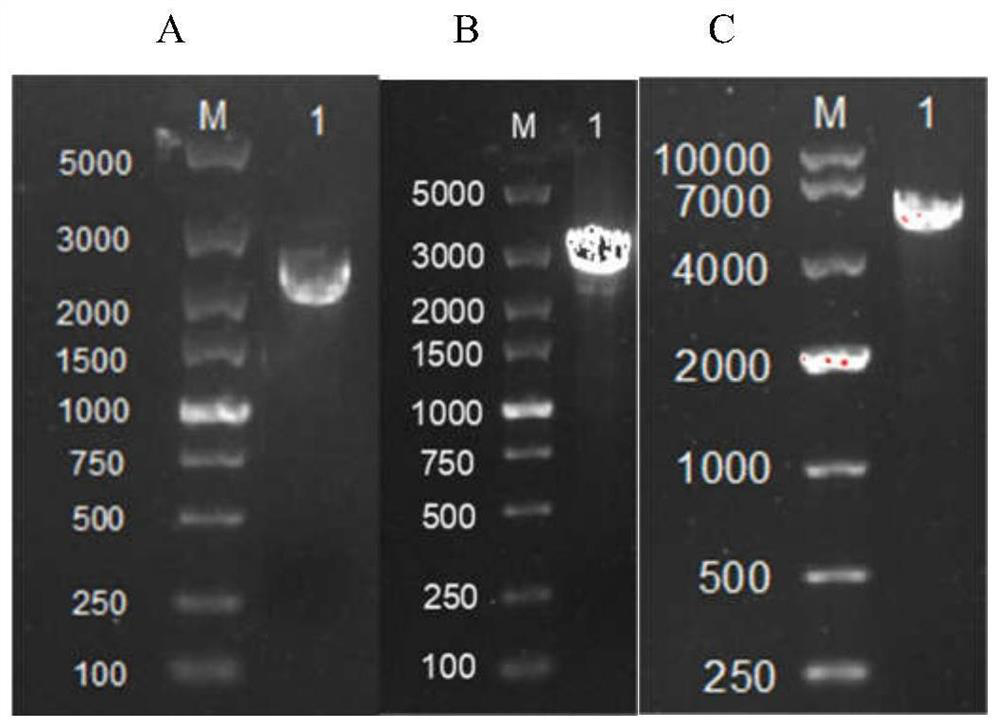
[0059] The size of the amplification product of the target gene HSF1 is 2441bp (SEQ ID NO.1), and the electropherogram is as attached figure 2 As shown in A. The PCR products were purified by TAKARA Gel Extraction Kit and used for subsequent exp...
Embodiment 2
[0077] Example 2: Construction of Pichia pastoris genetically engineered bacteria overexpressing the HSF1 gene.
[0078] 1. Transformation of recombinant plasmids
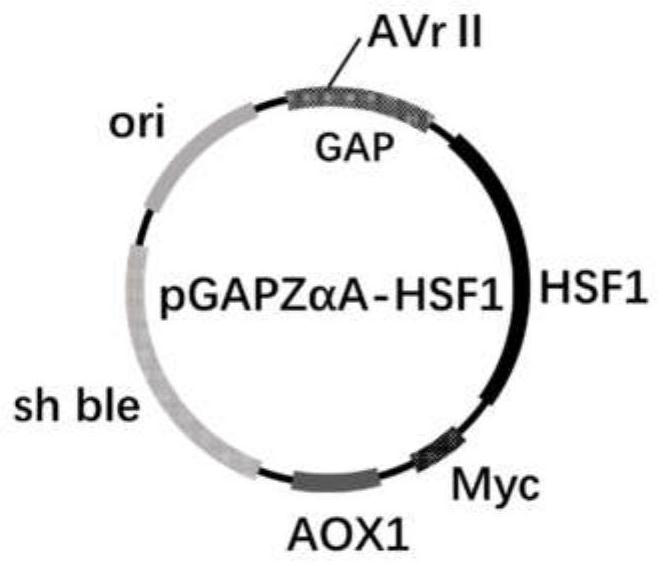
[0079] The plasmid pGAPZαA-HSF1 was digested and linearized with AvrII.
[0080] See Table 4 for the linearization system of the recombinant plasmid pGAPZαA-HSF1.
[0081] Table 4 Linearization system of recombinant plasmid pGAPZαA-HSF1
[0082] components Volume (μL) Manufacturer QuickCut AvrⅡ 1 TAKARA BIO INC. 10*QuickCut Buffer 5 TAKARA BIO INC. Recombinant plasmid pGAPZαA-HSF1 4 sterile water 40 total 50
[0083] The enzyme digestion condition was 37°C for 1 hour. After the enzyme digestion reaction, the enzyme digestion products were recovered from the gel and used for subsequent experiments.
[0084] The above-mentioned linearized recombinant plasmid pGAPZαA-HSF1 was introduced into Pichia pastoris P. Pastoris GS115 competent cells (purchased from M...
Embodiment 3
[0092] Example 3: Characterization and detection of biofilm formation.
[0093] Such as Figure 4 As shown, A and B are the results of the SEM electron microscopy experiments of the starting bacteria P. Pastoris GS115 and the recombinant strain GS115-HSF1*, respectively. It can be clearly seen that the film-forming effect of the recombinant strain is better than that of the starting bacteria, and the biofilm A large amount is formed.
[0094] Figure 5 It is a semi-quantitative biofilm measurement experiment conducted by crystal violet staining. Add 20 μL of starting bacteria and recombinant bacteria to a 96-well plate with 200 μL of YPD in each well, and culture it for 72-120 hours. After 12 hours, its OD570 was measured by crystal violet staining and microplate reader. It can be seen from the figure that the film-forming effect of the recombinant strain GS115-HSF1* is better than that of P. Pastoris GS115.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com