Porcine antibacterial peptide PMAP-23 variant and application thereof in preparation of feed
A PMAP-23, antimicrobial peptide technology, applied in the direction of cationic antimicrobial peptides, applications, antibacterial drugs, etc., can solve the problems of low yield of natural antimicrobial peptides, small yield of antimicrobial peptides, and high separation difficulty, and achieves improved growth performance and increased inhibition. Bacterial activity and safety, hemolysis rate reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
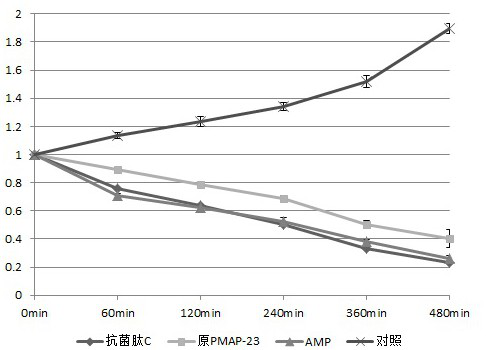
[0032] Embodiment 1: preparation of antibacterial peptide, antibacterial activity test and bactericidal kinetic test
[0033] (1) Design and synthesis of antimicrobial peptides:
[0034] On the basis of the original porcine antimicrobial peptide PMAP-23, some amino acids of the antimicrobial peptide were modified, and the antimicrobial peptide with the following amino acid sequence was obtained:
[0035] Antimicrobial peptide A: mutate the 6th position of PMAP-23 from leucine (L) to lysine (K) to obtain antimicrobial peptide A with the amino acid sequence of SEQID No: 3;
[0036] SEQ ID NO: 3: N-RIIDLKWRVRRPQKPKFVTVWVR-C
[0037] Antimicrobial peptide B: mutate the 6th position of PMAP-23 from leucine (L) to lysine (K), and from 20th position of valine (V) to cysteine (C) to obtain the amino acid sequence Antimicrobial peptide B of SEQ ID No: 4;
[0038] SEQ ID NO: 4: N-RIIDLKWRVRRPQKPKFVTCWVR-C
[0039] Antimicrobial peptide C: mutate the 6th leucine (L) of PMAP-23 to l...
Embodiment 2
[0054] Embodiment 2: acid-base stability, thermostability test
[0055] (1) Acid-base stability test
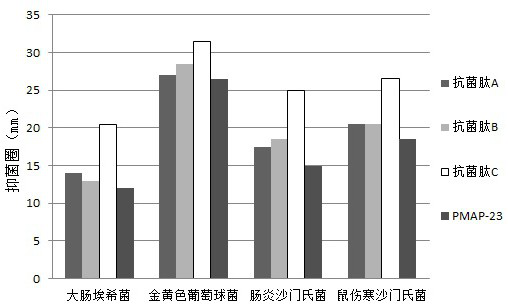
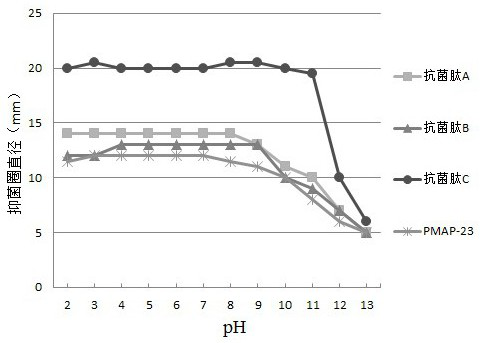
[0056] To test the effect of pH value, temperature and salt on the antibacterial activity of antimicrobial peptides, ddH was prepared with HCl and NaOH 2 O, so that the pH values are 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, ddH with different pH values will be prepared 2 O Dissolve antimicrobial peptides A-C and original PMAP-23, according to the method of "(3) antibacterial activity test" in Example 1, use Escherichia coli as a marker bacterium, observe the impact of different treatments on antibacterial peptide antibacterial activity, For specific results, see image 3 shown.
[0057] based on image 3 The displayed results show that the activity of the original PMAP-23 decreases when the pH exceeds 7, and its activity basically disappears when the pH reaches 13; When the pH was 12, the antimicrobial peptide C of the present invention showed an obvious decline in ac...
Embodiment 3
[0061] Embodiment 3: hemolytic activity test, feeding safety test
[0062] In order to evaluate the safety of antimicrobial peptides for animals, a hemolytic activity test and feeding safety test were carried out. The specific test plan is as follows:
[0063] (1) Hemolytic activity test
[0064] The defibrated pig blood was centrifuged at 1000 rpm for 10 min, the supernatant was discarded, and the red blood cells were repeatedly washed with PBS until the supernatant was clear. Resuspend red blood cells with 10 times the volume of PBS to prepare red blood cell suspension. The antimicrobial peptide antimicrobial peptide C and the original PMAP-23 antimicrobial peptide were serially diluted with PBS according to the doubling dilution method, and diluted to concentrations of 10240, 5120, 2560, 1280, 640, 320, 160, 80, and 40 μg / mL, respectively. Take 10 μL antimicrobial peptide solution and add it into the 96-well plate as the experimental group and control group, and take 10 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com