Preparation method of brivaracetam intermediate
A technology of intermediates and volumes, applied in the field of medicine, can solve the problems of difficult purification of isomers, achieve the effects of shortening process time, reducing process cost, obvious separation effect and cost advantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
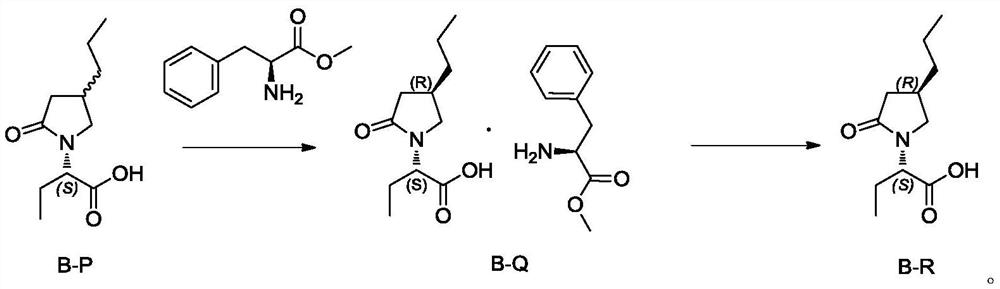
[0034] Embodiment 1: the preparation of compound B-P
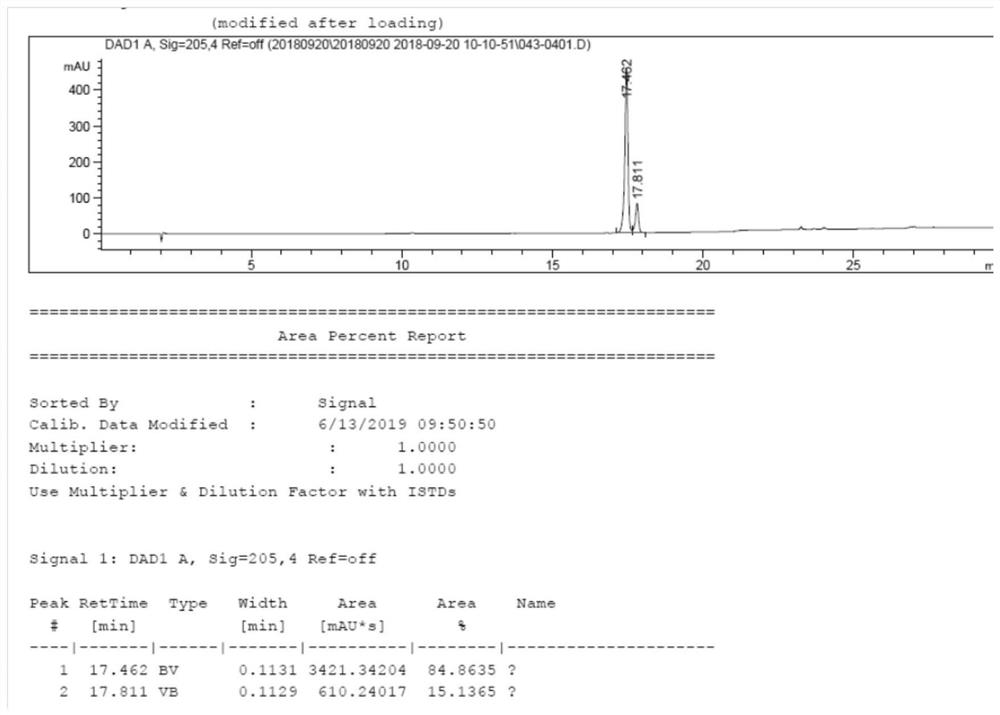
[0035] Add 150g of (S)-2(4-propyl-1,5-dihydropyrrol-2-one)butanoic acid (0.71mol) into a 2L hydrogen autoclave, add 1.5L of methanol, add 1.5g of Pd(OH ) 2 / C (1%), reacted at a pressure of 15-20 MPa for 20 hours, filtered, concentrated and distilled off methanol to obtain 140 g of compound B-P as a solid, RS:SS=85:15. See the attached HPLC chart figure 1 .
Embodiment 2
[0036] Embodiment 2: the preparation of L-phenylalanine methyl ester
[0037] Add 20gL of phenylalanine methyl ester hydrochloride to 100ml of methanol to dissolve, adjust the pH to 8-9 with 7N ammonia / methanol solution, filter with suction, and concentrate the mother liquor to obtain 17g of oil.
Embodiment 3
[0038] Embodiment 3: the preparation of compound B-Q
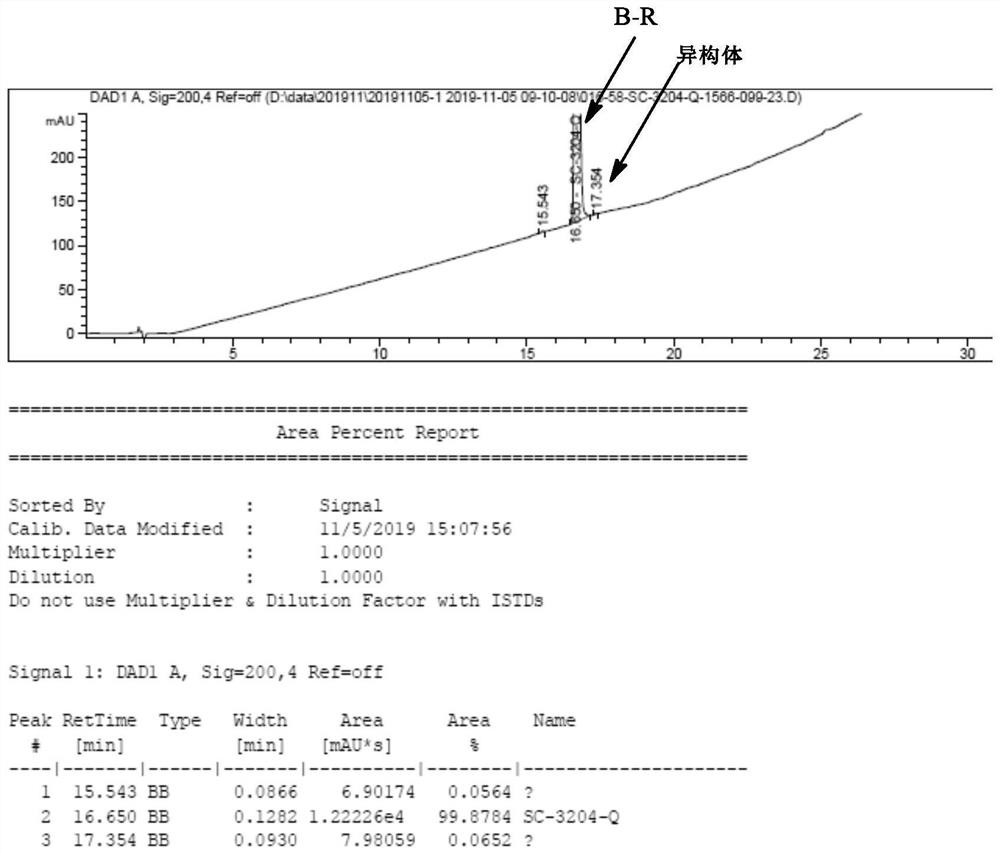
[0039] Add 18ml acetonitrile (3v) and 6g compound B-P (0.028mol, containing isomer 15%) and 5g L-phenylalanine methyl ester (1.0eq, 0.028mol) in 50ml there-necked flask, stir at 50 ℃ for 1 hour After cooling, low-temperature suction filtration, 10g of wet product was obtained, and dried to obtain 8g of compound B-Q. Yield: 73%, purity: 99.8%, diastereomers 0.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com