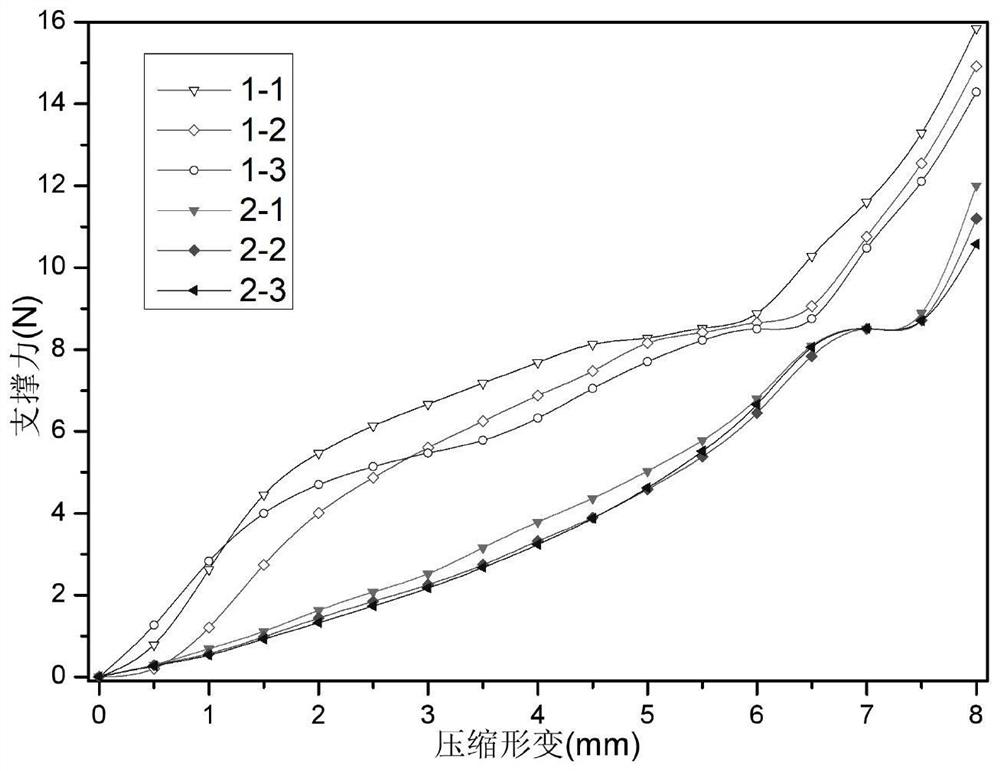
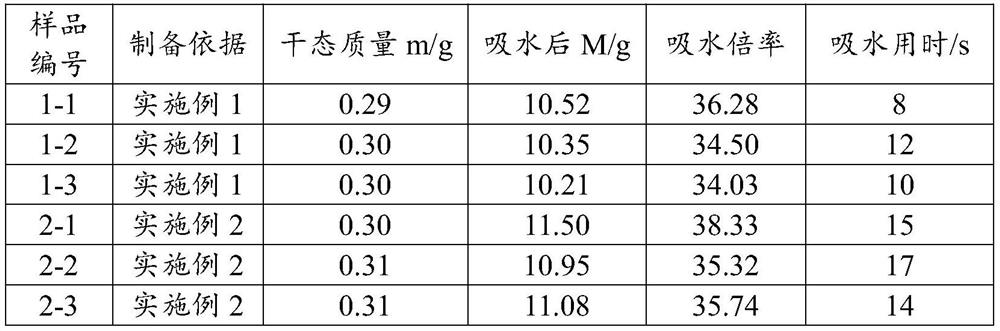
Degradable hemostatic sponge as well as preparation method and application thereof and degradable drug-loaded hemostatic sponge
A hemostatic sponge and drug-loading technology, which can be used in applications, drug delivery, and pharmaceutical formulations. It can solve the problems of slow water absorption, polyurethane residue, and poor support strength of the hemostatic sponge, and achieve excellent biocompatibility and water-locking ability. Strong, fast water absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In the present invention, the preparation method of the cross-linked modified starch preferably comprises the following steps:
[0044] Mixing starch and water for gelatinization reaction to obtain a gelatinized starch solution;
[0045] The gelatinized starch solution is mixed with a cross-linking agent to carry out a cross-linking reaction to obtain a cross-linked modified starch.
[0046] The invention mixes starch and water to carry out gelatinization reaction to obtain a gelatinized starch solution. In the present invention, the starch is preferably one or more of potato starch, tapioca starch, corn starch, sweet potato starch and wheat starch, more preferably potato starch or corn starch; in the present invention, the starch and The mass concentration of the starch solution obtained after water mixing is preferably 0.5-20%, more preferably 1.5-15%, most preferably 2.5-12%; the present invention has no special requirements for the specific implementation process o...
Embodiment 1
[0086] Weigh 100.0g of potato starch into the reactor, add 4900ml of water into the reactor, set the temperature of the jacketed reactor to 80°C, and stir at 200rpm for 1 hour; reduce the temperature of the jacketed reactor to 50°C, and use a concentration of 1mol / L NaOH solution to adjust the pH of the reaction solution to 9, use a constant pressure titration funnel to add 10ml of epichlorohydrin aqueous solution dropwise to the reaction solution, and react at a constant temperature for 4 hours. After the reaction is completed, the reaction solution is poured out. Precipitate the product with 1 L of absolute ethanol, wash the product 2 to 3 times with 2 L of absolute ethanol, and dry at 50°C for 24 hours to obtain cross-linked modified potato starch;
[0087] Weigh 1.0g carboxymethylcellulose into 39ml water, stir at 400rpm in a water bath at 40°C for 4 hours to completely dissolve carboxymethylcellulose into a uniform transparent gel, and use 1mol / L HCl to adjust the pH of ca...
Embodiment 2
[0094] Cross-linking modified potato starch synthetic method is with embodiment 1;
[0095] Weigh 0.5g of hydroxyethyl cellulose into 29.5mL of purified water, put it in a water bath at 50°C and stir at 400rpm for 2 hours to completely dissolve the hydroxyethyl cellulose into a uniform transparent gel, and use 1mol / L HCl to adjust the hydroxyethyl cellulose The pH of the solution was 3, cooled to room temperature for subsequent use;
[0096] Weigh 2.5g of cross-linked potato starch into 67.5mL of purified water, stir to absorb water and pour into hydroxyethyl cellulose solution, stir at 400rpm for 30min to mix evenly, after mixing evenly, use 1mol / L HCl to adjust the pH of the solution to 6 , put it in a centrifuge tube, set the centrifugation speed at 5000rpm, and centrifuge for 10min to remove the air bubbles in the solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com