Method for electrochemical synthesis of isocoumarin compound
An isocoumarin and compound technology, applied in electrodes, electrolysis components, electrolysis process and other directions, can solve the problems of poor C-H bond selectivity of aryl carboxylic acid and a large number of oxidants, and achieve the effect of good purity and high yield
Inactive Publication Date: 2021-06-15
HENAN NORMAL UNIV
View PDF4 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0006] In order to overcome the existing problems in the prior art: 1) weakly coordinated aryl carboxylic acid C-H bond selectivity is poor, 2) defects such as the use of a large amount of oxidant, the invention discloses a novel isocoumarin compound Preparation method; the preparation method can obtain the cyclization product by C-O / O-H bond with high selectivity under the condition of electric anodic oxidation and under the catalysis of transition metal iridium; the method has mild reaction conditions, environment-friendly, high yield, good purity, suitable for industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0026] The present invention is further illustrated below by means of examples, but the present invention is not limited to the scope of the examples. For the experimental methods that do not specify specific conditions in the following examples, select according to conventional methods and conditions, or according to the product instructions.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
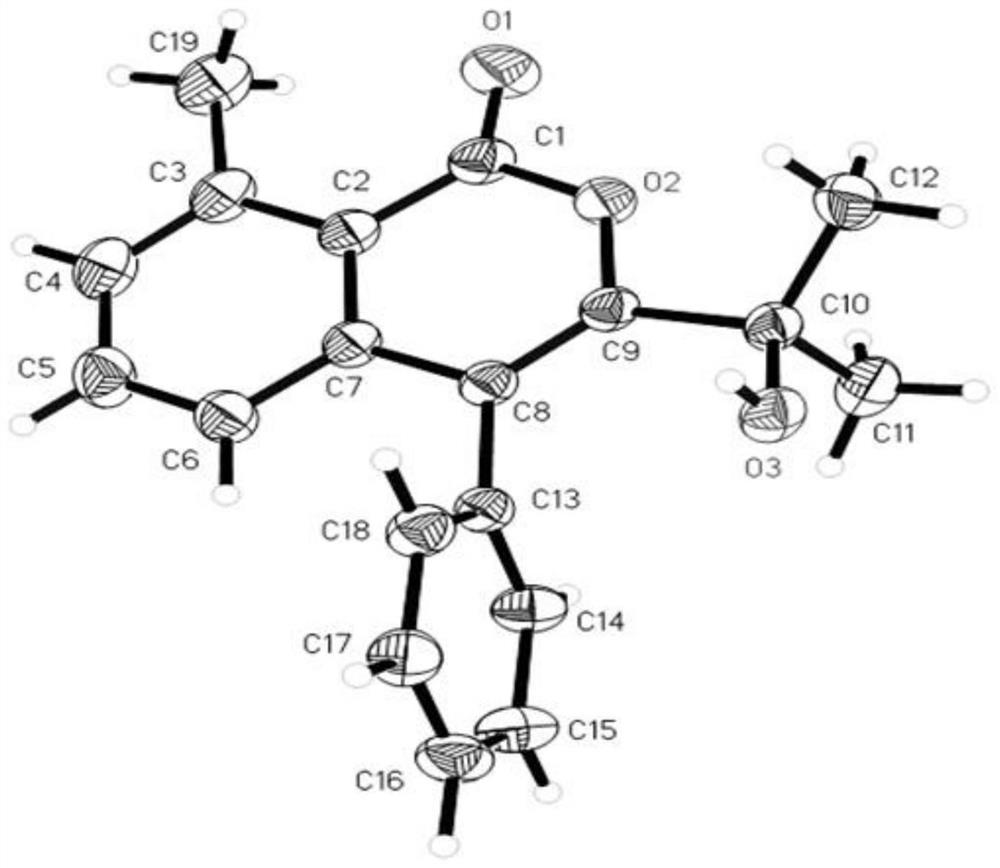
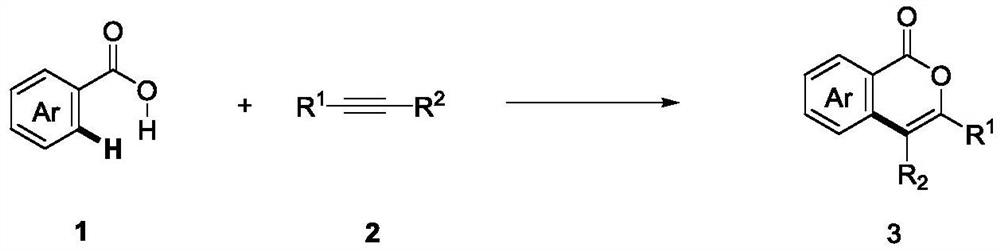
The invention discloses a method for synthesizing an isocoumarin compound through electrochemical oxidative dehydrogenation coupling, and belongs to the technical field of organic chemistry. In a non-separated electrolytic tank, substituted aryl formic acid 1 and an alkyne compound 2 are subjected to a constant-current electrolytic reaction in an organic solvent in the presence of an iridium catalyst and an additive to obtain an isocoumarin derivative 3. According to the method disclosed by the invention, a C-O / O-H bond cyclization product is obtained with high selectivity under the condition of electric anodic oxidation and the catalysis of transition metal iridium. The method is mild in reaction condition, environment-friendly, high in yield and good in purity, and a simple and effective synthesis way is provided for the isocoumarin compound.
Description
technical field [0001] The invention relates to a preparation method of isocoumarin-containing derivatives, in particular to a method for synthesizing isocoumarin compounds through electrochemical oxidative dehydrogenation coupling, and belongs to the field of organic chemistry. Background technique [0002] Isocoumarins are an important class of natural lactones that exist in a variety of natural products and have various biological activities, such as antifungal, antitumor, antiallergic, antimicrobial, anti-inflammatory and anticancer activities. Isocoumarins are also essential intermediates in the synthesis of many other compounds such as isoquinolines, isocarbitin and isochromones. [0003] Traditional methods for the synthesis of such compounds employ transition metal-catalyzed cyclization of ortho-halogenated aromatic esters or carboxylic acids with π-electron components. Among the methods reported in the existing literature, transition metal (Rh, Ir, Ru) catalyzed di...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C25B3/05C25B3/07C25B3/23C25B11/081
Inventor 杨启亮刘颖贾红伟渠桂荣郭海明
Owner HENAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com