Immune activation antibody and application thereof
An immune activation, antibody technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc., can solve the problems of normal tissue damage and side effects, and achieve the effect of avoiding damage and side effects and having a good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0155] The synthetic method of compound 1, comprises the steps:
[0156] Dissolve 2.37 g of compound Pro-1 and 1.2 g of propyne bromide in 50 mL of DMF, and add 1.5 g of K 2 CO 3 , and stirred at room temperature for 12 hours. After filtration, the filtrate was distilled under reduced pressure (less than 60°C) to remove the solvent, and the residue was added with 25 mL of concentrated hydrochloric acid, and stirred at room temperature for 10 hours. reactant with K 2 CO 3 Neutralize to pH 7, precipitate a crude solid product, dissolve in methanol, separate by silica gel column chromatography (methanol: dichloromethane = 1:10 volume ratio), collect the product solution, concentrate under reduced pressure to remove the solvent, and obtain a white solid product which is the compound 1. ESI-MS: m / z=263.10[M+H] + .
[0157] The reaction formula of synthetic compound 1 by compound Pro-1 is as follows:
[0158]
[0159] The synthesis method of compound 2 and compound 3 is ba...
Embodiment 1
[0330] TLR7 Activation Detection of the Compound of Example 1 (HEK-BlueTM Detection)
[0331] Take HEK-Blue TMh TLR7 cells in the logarithmic growth phase (purchased from InvivoGen), discard the growth medium (Gibco, C11995500BT, Invivo Gen, ant-nr), and take an appropriate amount of 37°C PBS (Hyclone, SH30256.01) Wash the cells twice by lightly rinsing, and discard the PBS. Add 2-5mL 37°C PBS, incubate for 1-2min, scrape off the cells with a cell scraper, and blow gently to disperse them into a single-cell suspension. The cells were counted and the cell concentration was calculated using a hemocytometer, and the cell suspension was adjusted to 2.5×104 / 180 μL per well using HEK-BlueTM Dectetion solution (purchased from Invivo Gen) for cell plating on a 96-well cell culture plate. HEK-BlueTM hTLR7 cells were stimulated according to the compound or drug concentration (for example, 0.01 μM, 0.1 μM, 1 μM, 5 μM, 15 μM, 30 μM, 40 μM), and three replicate wells were set for each con...
Embodiment 2
[0332] Example 2 Detection of the TLR7 agonist release effect of each immune-activating antibody
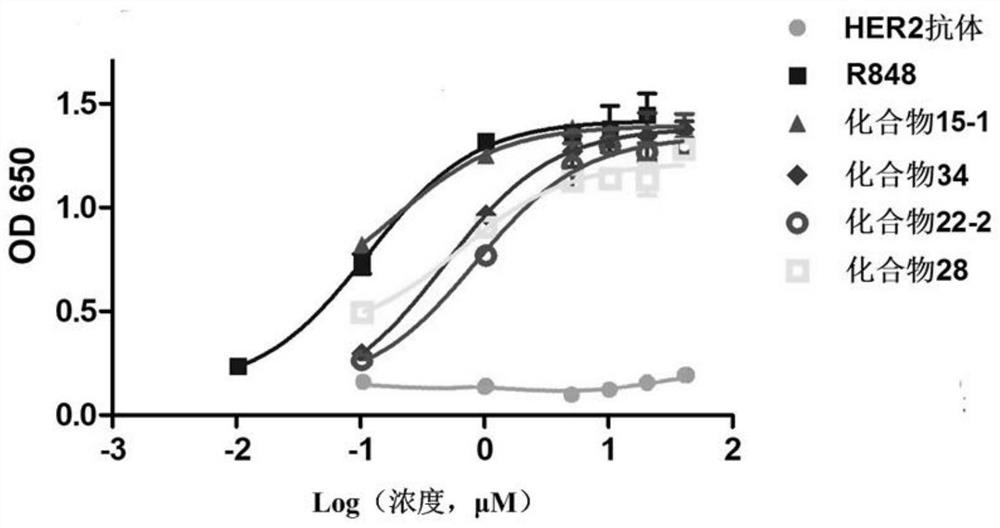
[0333] Take each immune-activating antibody and add it to HER2-positive SKBR3 (10 5 1) cells in RPMI1640 medium. After mixing and culturing for 12 hours, each supernatant was taken out, and the TLR7 activation effect was tested according to the method in Example 1. The result is as figure 2 with image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com