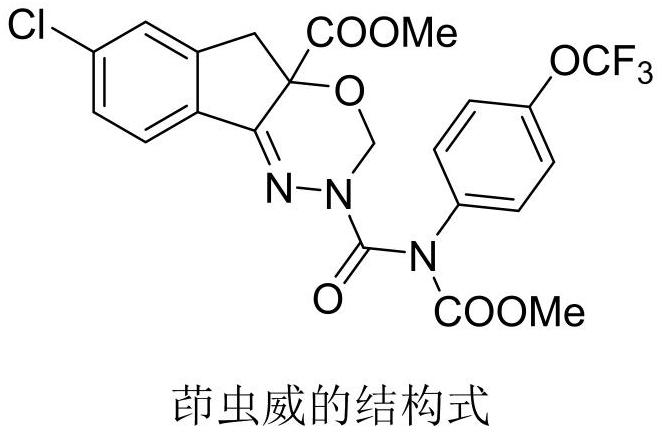
A kind of improved process method for preparing 5-chlorindanone
A technology of chlorindanone and process improvement, applied in the field of organic synthesis, can solve the problems such as no public refining method, and achieve the effects of shortening production time, reducing dosage, and good product color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
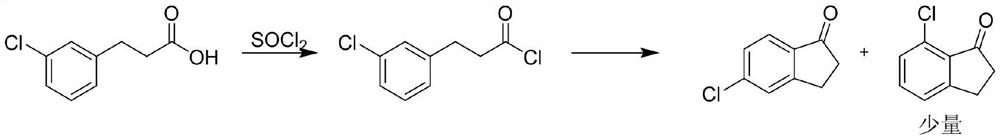
[0035] Embodiment 1 (traditional process)
[0036]
[0037] Put 225g (2.0mol, 1eq) of chlorobenzene into the reaction flask, cool down to 25-30°C, add 333g (2.5mol, 1.25eq) of aluminum trichloride under stirring, and slowly add dropwise at a temperature of 25-30°C 254g (2.0mol, 1eq) of 3-chloropropionyl chloride was added dropwise and kept for 3 hours. 466.7 g (3.5 mol, 1.75 eq) of aluminum trichloride, 163 g of sodium chloride and 23.3 g of potassium chloride were added to the molten state, and heated to 135° C. to react for 4 h. Sampling HPLC to detect no intermediate remaining, the reaction solution was added to 3700g of ice water for hydrolysis, filtered and dried to obtain 330g of black solid, which was added to 1000mL of methanol to dissolve and refluxed, hot filtered, the filtrate was heated to reflux again, 30g of activated carbon was added, hot filtered, and the filtrate was Cooling and crystallization yielded 242.9 g of yellow solid, yield 72.9%, HPLC 99.1%. 1 H...
Embodiment 2
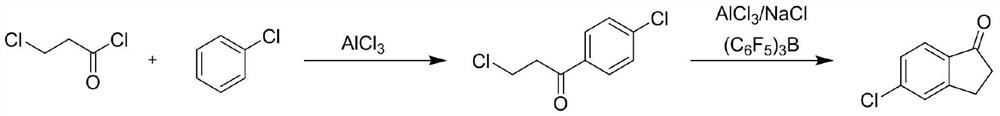
[0038] Embodiment 2 (traditional process)
[0039]
[0040] Put 227.4g (2.02mol, 1.01eq) of chlorobenzene into the reaction flask, cool down to 25-30°C, add 314.7g (2.36mol, 1.18eq) of aluminum trichloride under stirring, and control the dropwise temperature after stirring for 10 minutes Slowly add 254g (2.0mol, 1eq) of 3-chloropropionyl chloride dropwise at 25-45°C, and heat to 70-80°C for 2 hours after the dropwise addition. Sampling GC detection raw material 3-chloropropionyl chloride remaining 0.5%. It was added to 514.7g (3.86mol, 1.93eq) of aluminum trichloride and 57.3g (0.98mol, 0.49eq) of sodium chloride in a molten state, and heated to 150-165° C. to react for 3h. Sampling was detected by HPLC and no intermediate remained. After the incubation, the temperature was lowered to 105-110 ° C. The reaction solution was added to 3700 g of ice water for hydrolysis, filtered and dried to obtain 330 g of black solid. The crude product 330 g was divided into 3 parts. First, ...
Embodiment 3
[0042]
[0043] Put 227.4g (2.02mol, 1.01eq) of chlorobenzene into the reaction flask, cool down to 25-30°C, add 314.7g (2.36mol, 1.18eq) of aluminum trichloride under stirring, and control the dropwise temperature after stirring for 10 minutes Slowly add 254g (2.0mol, 1eq) of 3-chloropropionyl chloride dropwise at 25-45°C, and heat to 70-80°C for 2 hours after the dropwise addition. Sampling GC detection raw material 3-chloropropionyl chloride remaining 0.5%. It was added to 514.7g (3.86mol, 1.93eq) of aluminum trichloride and 57.3g (0.98mol, 0.49eq) of sodium chloride in a molten state, and heated to 150-165° C. to react for 3h. Sampling was detected by HPLC and no intermediate remained. After the incubation, the temperature was lowered to 105-110° C. and added dropwise to 2000 g of 4% hydrochloric acid prepared in advance. The dropwise temperature was controlled at 90-95° C., stirred for 10 minutes, allowed to stand for 30 minutes, and the lower organic layer was separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com