Preparation method of sugammadex sodium injection
A technology of sugammadex sodium injection, which is applied in the field of preparation of sugammadex sodium injection, to achieve the effects of increasing safety, reducing catalysis, and inhibiting the production of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
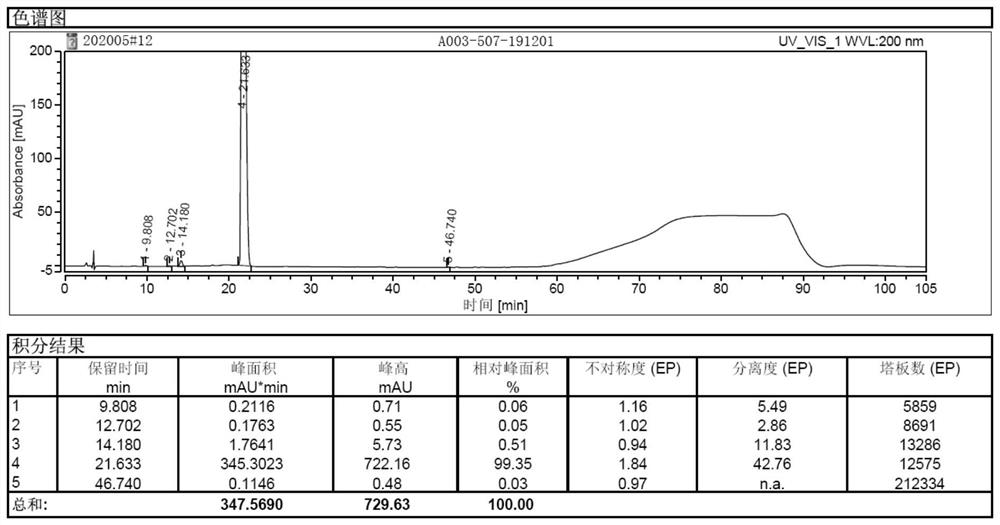
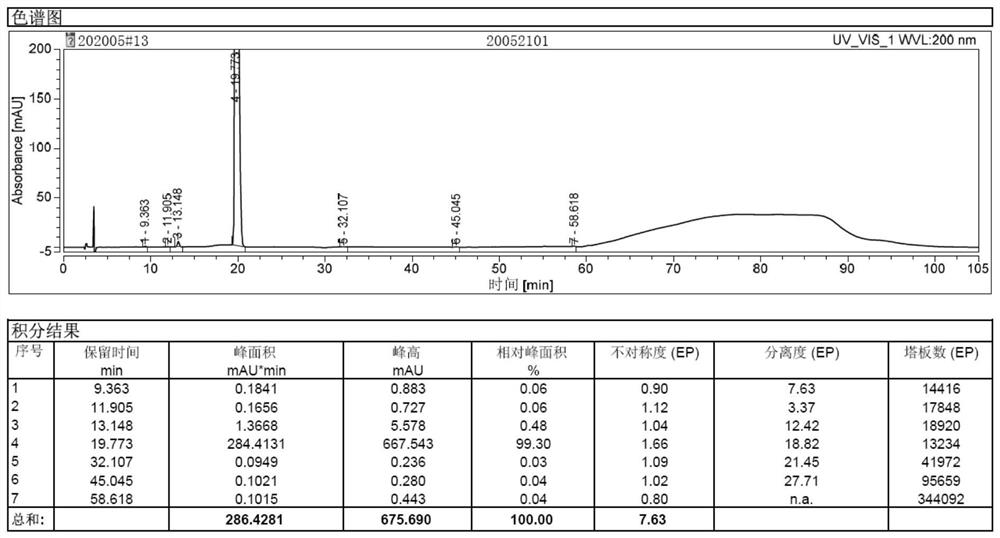
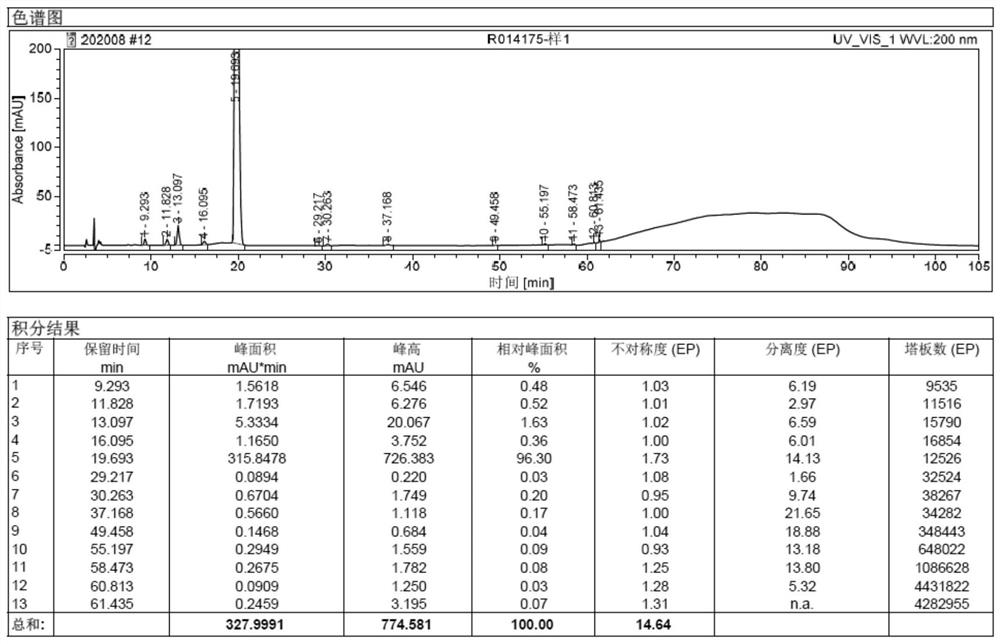
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of sugammadex sodium injection
[0044] Sugammadex Sodium Injection is commercially available in specifications of 200mg: 2ml and 500mg: 5ml. The injections of the two specifications are only different in filling volume and packaging material size. All the examples in the present invention are based on the specification of 200mg: 2ml The preparation process of the prescription is described as an example.
[0045] 1. Prescription
[0046]
[0047] 2. Process steps
[0048] (1) Add 80% of the prescribed amount of water for injection into the liquid mixing tank, fill with 99.999% of clean nitrogen, stir, and monitor the dissolved oxygen of the water for injection on-line;
[0049] (2) When the dissolved oxygen in water for injection reaches below 0.5mg / L, stop stirring and nitrogen filling, add the prescribed amount of sugammadex sodium raw material into the liquid mixing tank, start stirring, and nitrogen filling, and wait until the raw ...
Embodiment 2
[0055] Embodiment 2: Preparation of Sugammadex Sodium Injection
[0056] 1, carry out experimental operation according to embodiment 1 prescription
[0057] 2. Process steps
[0058] (1) Add 80% of the prescribed amount of water for injection into the liquid mixing tank, fill with 99.999% of clean nitrogen, stir, and monitor the dissolved oxygen of the water for injection on-line;
[0059] (2) When the dissolved oxygen in water for injection reaches below 0.5mg / L, stop stirring and nitrogen filling, add the prescribed amount of sugammadex sodium raw material into the liquid mixing tank, start stirring, and nitrogen filling, and wait until the raw material drug is completely dissolved , add inositol hexaphosphate 100g, fill with nitrogen and stir;
[0060] (3) Coarsely filter the liquid medicine, then pass through the GE8040F30 reverse osmosis membrane under nitrogen pressure, add water for injection to 95% of the total amount, and use hydrochloric acid or sodium hydroxide so...
Embodiment 3
[0066] Embodiment 3: Preparation of Sugammadex Sodium Injection
[0067] 1, carry out experimental operation according to embodiment 1 prescription
[0068] 2. Process steps
[0069] (1) Add 80% of the prescribed amount of water for injection into the liquid mixing tank, fill with 99.999% of clean nitrogen, stir, and monitor the dissolved oxygen of the water for injection on-line;
[0070] (2) When the dissolved oxygen in water for injection reaches below 0.5mg / L, stop stirring and nitrogen filling, add the prescribed amount of sugammadex sodium raw material into the liquid mixing tank, start stirring, and nitrogen filling, and wait until the raw material drug is completely dissolved , add 500 g of inositol hexaphosphate, fill with nitrogen and stir;
[0071] (3) Coarsely filter the liquid medicine, then pass through the GE8040F30 reverse osmosis membrane under nitrogen pressure, add water for injection to 95% of the total amount, and use hydrochloric acid or sodium hydroxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com