A kind of silicon-containing condensed pentacyclic bridged organic dye and its preparation method and application
A technology of organic dyes and reactions, applied in organic dyes, silicon organic compounds, organic chemistry, etc., to achieve high optical coefficient, inhibit interface electron recombination, and prevent self-aggregation of dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
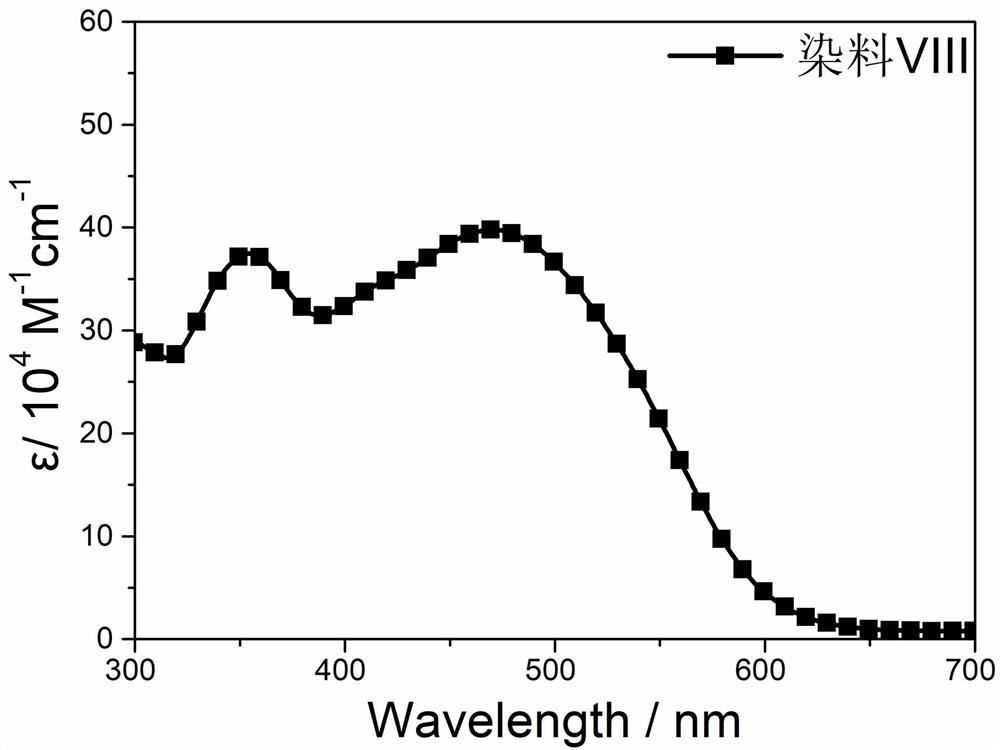
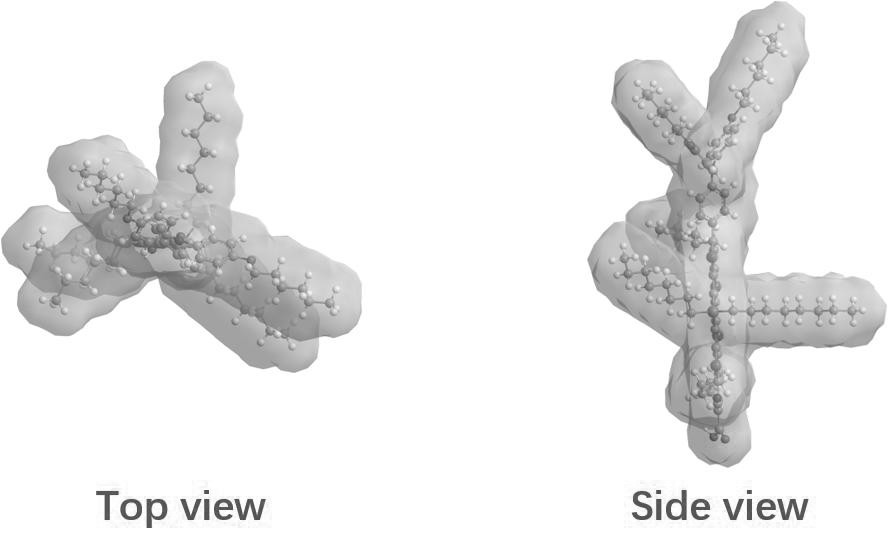
[0034] This embodiment provides a silicon-containing fused pentacyclic organic dye, the chemical formula of which is shown in formula (VIII)
[0035]
[0036] The preparation method of above-mentioned organic dye (formula (VIII) compound) is as follows:
[0037] The compound of formula (1) used in this example is based on literature Wang Z, Miu L, Yao H, et al.Organicsensitizers featuring 9H-thieno[2′,3′:4,5]thieno[3,2-b]thieno[ 2',3':4,5]thieno[2,3-d]pyrrole core for high performance dye-sensitized solarcells.Dyes Pigments 2019;162:126-35. Prepared; the compound of formula (6) is obtained according to document O. Bettucci, T. Skaltsas, M. Calamante, et al. ACS Appl. Energy Mater. 2019, 2, 5600-561. Prepared; other reagents can be obtained commercially.
[0038] S1: react the compound of formula (I) with the compound of formula (II) to generate the compound of formula (III);
[0039]
[0040] This step is specifically: under argon protection, under the condition of low...
Embodiment 2
[0060] This embodiment is roughly the same as Embodiment 1, and the main differences are:
[0061] Synthesis of compound of formula (III):
[0062] Under argon protection and low temperature -78°C, 1.47g of the compound of formula (I) dissolved in 30mL of anhydrous tetrahydrofuran was added to a 100mL eggplant-shaped reaction flask. After 5 minutes of low temperature reaction, 2.34mL of n-butyllithium was slowly added. (2.4mol / L), 794mg of compound (II) was added after warm reaction for 1h, the refrigeration was turned off, and slowly returned to room temperature; the reaction was performed for 12h. Water and ethyl acetate were added for extraction, the organic phase was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 782 mg of compound (III) as a yellow-green viscous liquid with a yield of 46%.
[0063] Synthesis of compound of formula (IV):
[0064] Add 600 mg of compound (III) to a 100 mL single-neck flask, dissol...
Embodiment 3
[0073] This embodiment is roughly the same as Embodiment 1, and the main differences are:
[0074] Synthesis of compound of formula (III):
[0075] Under argon protection, under the condition of low temperature -78 ℃, into a 100 mL eggplant-shaped reaction flask, add 981 mg of the compound of formula (I) dissolved in 30 mL of anhydrous tetrahydrofuran, and after 5 minutes of low temperature reaction, slowly add n-butyllithium 1.56 mL ( 2.4mol / L), after warm reaction for 1h, add 794mg of compound of formula (II), turn off refrigeration, slowly return to room temperature; react for 8h. Water and ethyl acetate were added for extraction, the organic phase was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 737 mg of the compound of formula (III). It was a yellow-green viscous liquid with a yield of 65%.
[0076] Synthesis of compound of formula (IV):
[0077] Add 600 mg of compound (III) to a 100 mL single-neck flask, d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com