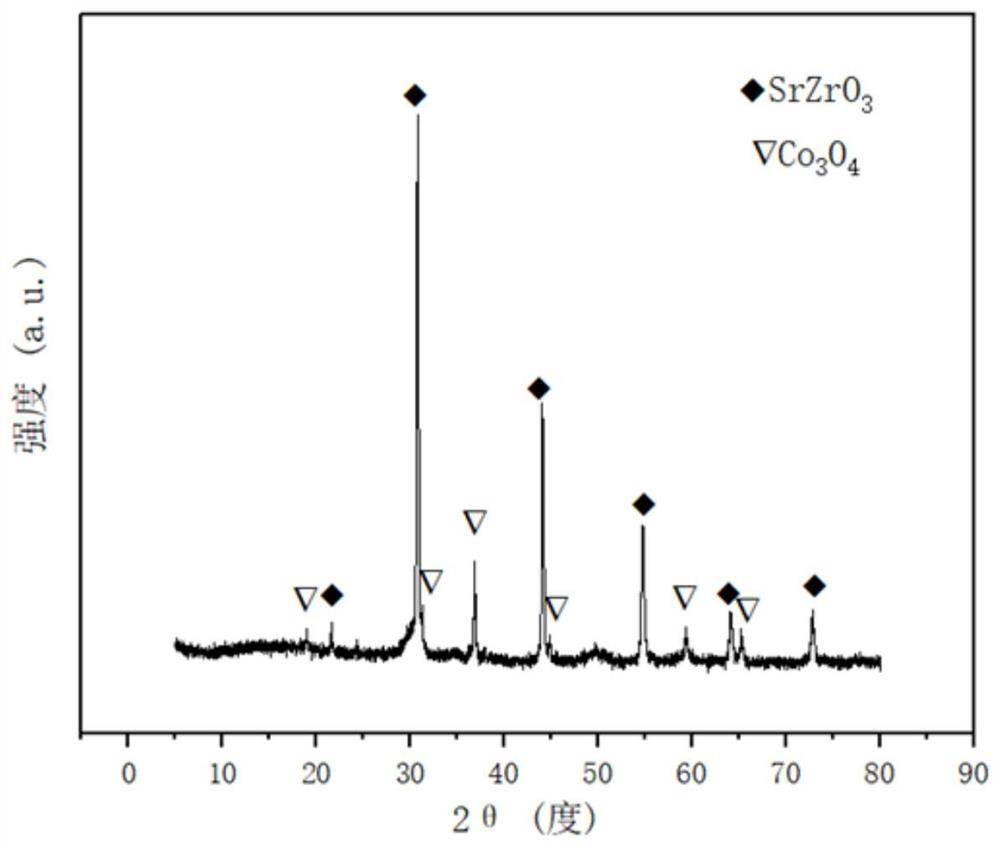
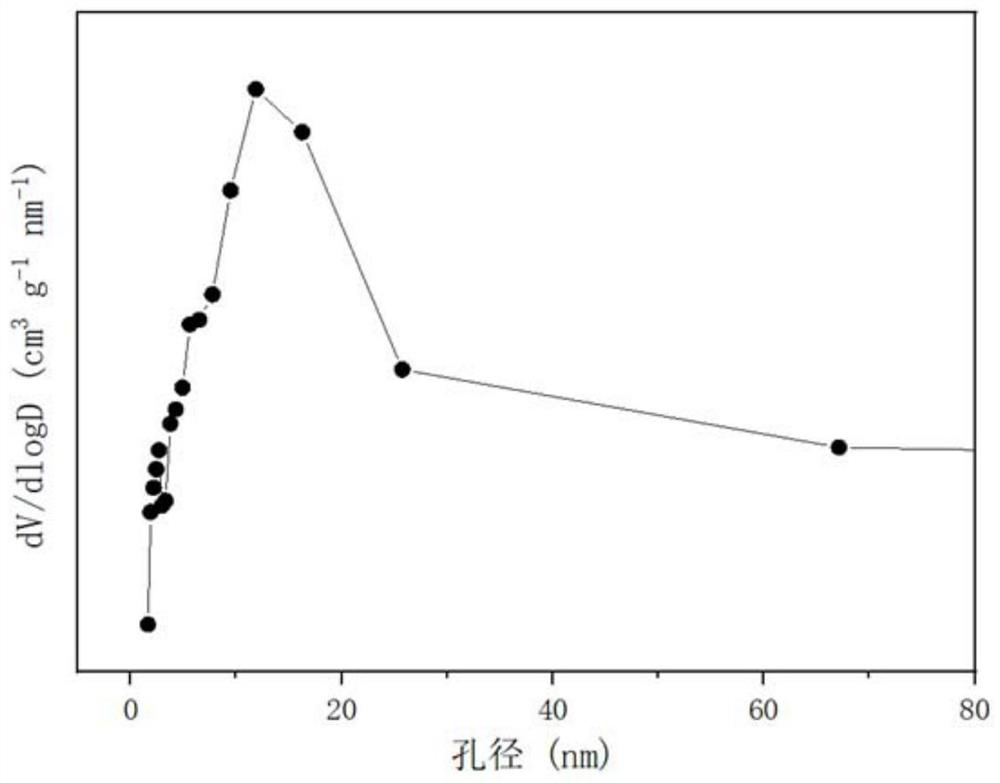
A strontium zirconium perovskite-type cobalt-based catalyst for hydrogen production by autothermal reforming of acetic acid
A cobalt-based catalyst, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, hydrogen, etc., can solve the problem of catalyst deactivation, to promote migration, reduce The amount of carbon deposition and the effect of improving alkalinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Weigh 3.667 g of zirconium n-propoxide, put it into a beaker containing 1.3 mL of triethanolamine, and stir and mix at room temperature for 24 h to form a stable zirconium-triethanolamine complex; under continuous stirring, 1.792 g of hexahydrate Cobalt nitrate and 5.7 mL of 1,3-propanediol were added to the above-mentioned complex, and stirred at room temperature for 90 min to obtain a mixed solution; this solution was transferred to a polytetrafluoroethylene-lined autoclave, and placed in a 140° C. In the oven for 72h; after the autoclave was cooled to room temperature, the precipitate was collected by filtration and washed with deionized water until neutral, and then the precipitate was placed in an oven at 105°C to dry for 12h; the sample was placed in a tube furnace for 10 The heating rate of ℃ / min was increased to 700 ℃, and the catalyst CDUT-ZC was obtained after calcination at this temperature for 4 h. The catalyst had a molar composition of oxides (ZrO 2 ) 1.8...
Embodiment 1
[0029] Weigh 3.667g of zirconium n-propoxide, put it into a beaker containing 1.3mL of triethanolamine, and stir and mix at room temperature for 24h to form a stable zirconium-triethanolamine complex; under continuous stirring, 2.974g of Sr (OH) 2 ·8H 2 O, 1.792g of cobalt nitrate hexahydrate and 5.7mL of 1,3-propanediol were added to the above-mentioned complex, and stirred at room temperature for 90min to obtain a mixed solution; this solution was transferred to a polytetrafluoroethylene-lined autoclave , and placed in an oven at 140°C for 72h; after the autoclave was cooled to room temperature, the precipitate was collected by filtration and washed with deionized water until neutral, and then the precipitate was placed in an oven at 105°C to dry for 12h; the sample was placed in In a tube furnace, the heating rate was raised to 700 °C at a heating rate of 10 °C / min, and after calcination at this temperature for 4 h, a CDUT-SZC catalyst was obtained, that is, a Co-containin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com