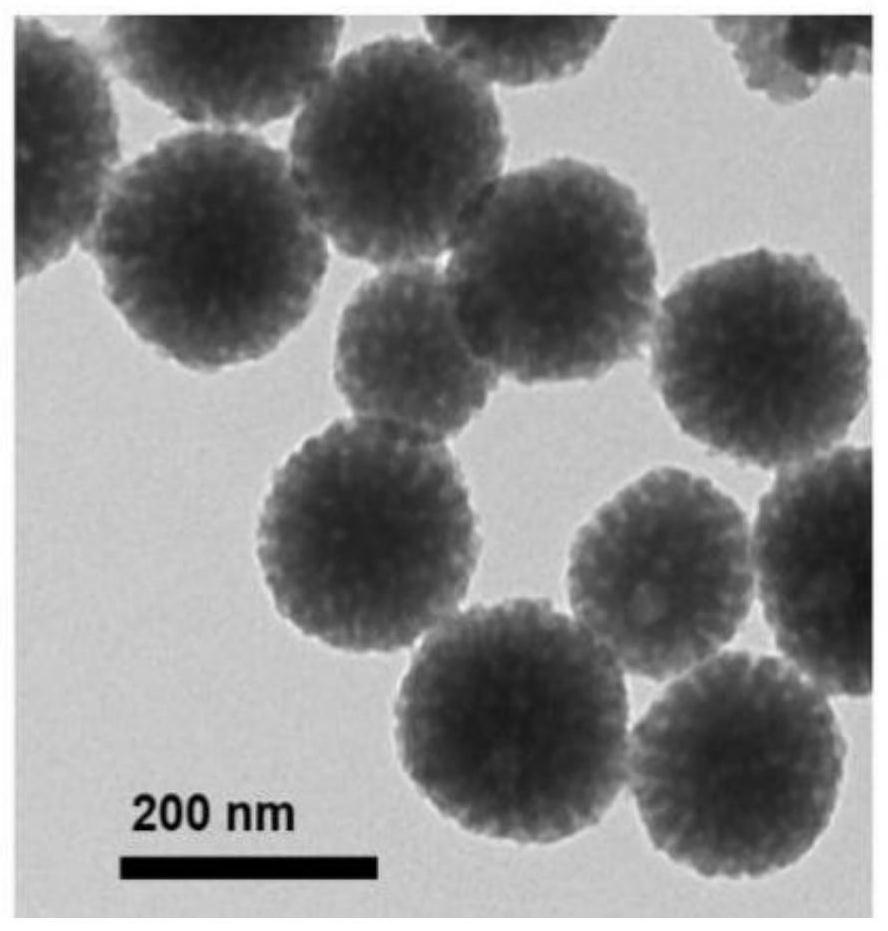
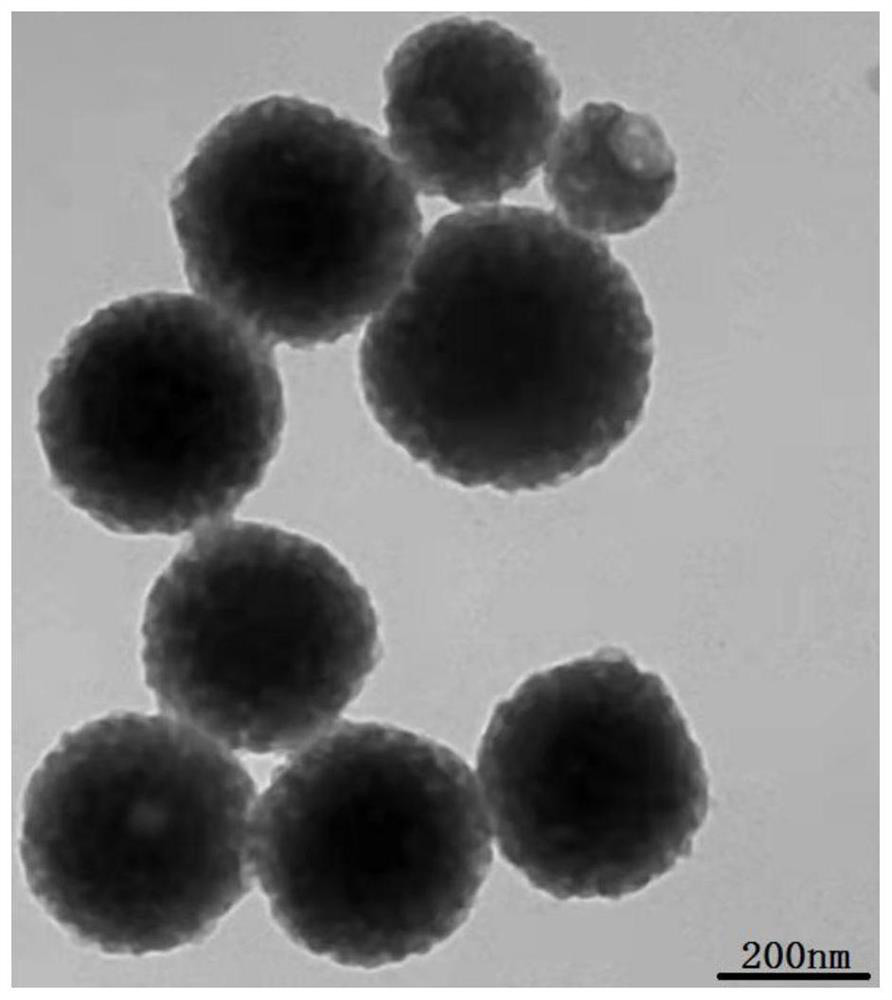
Mesoporous polydopamine nanoparticles for tumor T1-T2 magnetic resonance imaging
A mesoporous polydopamine and nanoparticle technology, applied in the field of biomedical materials, can solve the problems of not having T1-T2 dual-mode imaging effect and limiting the application of tumor diagnosis and treatment, and achieve good photothermal conversion ability, accurate MRI results, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of manganese-mesoporous polydopamine nanoparticles by doping method before polymerization
[0052] (1) Dissolve 90 mg of dopamine hydrochloride and 3 mg of manganese sulfate hydrate in 3 mL of ultrapure water (the mass ratio of dopamine hydrochloride to manganese sulfate hydrate is 30:1; the mass-volume ratio of manganese sulfate hydrate to ultrapure water is 1 mg:1 mL) , Stir at 180rpm in the dark for 24h to obtain a manganese chelate-dopamine solution;
[0053] (2) the 0.3mL manganese chelation-dopamine solution of 36mg surfactant F127 and step (1) is joined in the mixed solution of 6mL ethanol and 6mL ultrapure water (the mass volume ratio of F127 and manganese chelation-dopamine solution is 120mg: 1mL, the volume ratio of ethanol, ultrapure water and manganese chelation-dopamine solution is 20:20:1), and stirred at 180rpm in the dark for 20min; figure 1 Shown in (1), manganese chelation-dopamine solution, F127 are dispersed in the mixed soluti...
Embodiment 2
[0057] Example 2 Preparation of manganese-mesoporous polydopamine nanoparticles by doping method before polymerization
[0058] (1) 99mg dopamine hydrochloride and 3mg manganese sulfate hydrate are dissolved in 3.75mL ultrapure water (the mass ratio of dopamine hydrochloride and manganese sulfate hydrate is 33:1; the mass volume ratio of manganese sulfate hydrate and ultrapure water is 1mg:1.25 mL), 150rpm avoid light and stir for 26h, obtain manganese chelation-dopamine solution;
[0059] (2) join the manganese chelation-dopamine solution of 30mg surfactant F127 and 0.3mL step (1) in the mixed solution of 4.8mL ethanol and 4.8mL ultrapure water (the mass volume of F127 and manganese chelation-dopamine solution Ratio is 100mg:1mL, the volume ratio of ethanol, ultrapure water and manganese chelation-dopamine solution is 20:20:1.25), 150rpm avoids light and stirs 25min;
[0060] (3) Add 0.3mL 1,3,5-trimethylbenzene (TMB) solution while shaking in a water bath ultrasonic (4kHz) ...
Embodiment 3
[0063] Example 3 Manganese-mesoporous polydopamine nanoparticles prepared by doping before polymerization
[0064] (1) 105mg dopamine hydrochloride and 3mg manganese sulfate hydrate are dissolved in 4.5mg ultrapure water (the mass ratio of dopamine hydrochloride and manganese sulfate hydrate is 35:1; the mass volume ratio of manganese sulfate hydrate and ultrapure water is 1mg:1.5 mL), and stirred at 200rpm in the dark for 22h to obtain manganese chelation-dopamine solution;
[0065] (2) join the 0.3mL manganese chelation-dopamine solution of 45mL surfactant F127 and step (1) in the mixed solution of 4mL ethanol and 4mL ultrapure water (the mass volume ratio of F127 and manganese chelation-dopamine solution is 150mg: 1mL, the volume ratio of ethanol, ultrapure water and manganese chelation-dopamine solution is 20:20:1.5), 150-200rpm dark stirring for 25min;
[0066] (3) Add 0.4mL 1,3,5-trimethylbenzene (TMB) solution while shaking in a water bath ultrasonic (4kHz) at 25°C, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrated particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com